Dutch researchers have concluded that whole-body PET with fluorine-18 fluoroestradiol (F-18 FES) can be a useful diagnostic tool in estrogen-receptor-positive breast cancer patients, according to a study published in the February issue of the Journal of Nuclear Medicine.
However, whole-body FES-PET is not a reliable technique to evaluate liver metastases: The modality missed 24% of liver lesions in the study, which was led by Dr. Michel van Kruchten from the department of medical oncology at University Medical Center Groningen (JNM, Vol. 53:2, pp. 191-199).
As the authors noted, approximately 75% of tumors express the estrogen receptor at the time of the patient's diagnosis. The additional information of a patient's estrogen-receptor status significantly affects her treatment regimen.
Gold standard
The gold standard for evaluating estrogen-receptor status has been immunohistochemical staining of the primary tumor. However, this technique can correctly predict tumor response to antihormonal therapy in only 50% to 60% of cases, the authors wrote.
In the current study, 33 women with a history of histologically proven estrogen-receptor-positive breast cancer were referred for FES-PET imaging between December 2008 and October 2010 after their standard workup was inconclusive.
Whole-body imaging was performed on either a PET (ECAT Exact HR1, Siemens Healthcare) or 64-slice PET/CT (Biograph mCT, Siemens) scanner 60 minutes after injection of the FES tracer. The researchers then recorded the number of lesions detected by FES-PET and the FES standardized uptake values (SUVs).
Lesions were classified as benign, equivocal, or metastatic, and conventional imaging results were compared with findings on FES-PET. For discordant lesions, a fusion of FES-PET with CT, MRI, or FDG-PET and a comparison between FES-PET and bone scans were performed to classify those lesions.
Physician questionnaire
Van Kruchten and colleagues also developed questionnaires for referring physicians to complete before, shortly after, and more than three months after FES-PET. One questionnaire was designed to provide details for the initial inconclusive result and potential therapeutic strategy. The second questionnaire evaluated the outcome of the FES-PET scan and how it affected a patient's treatment. The third questionnaire addressed how FES-PET aided a patient's diagnosis and the management of therapy after a follow-up of least three months.
Patients also were retrospectively divided into three groups based on the reason for their respective FES-PET exams. For the first group of 21 patients, the exam was to differentiate between benign and malignant lesions in the case of equivocal or inconclusive workup. In the second group, which included 10 patients, the exam was to evaluate estrogen-receptor status, and in the third group, consisting of two patients, the exam was to distinguish between metastases from different tumor types.
A total of 22 patients received FES-PET scans, while 11 patients underwent FES-PET/CT. Workup prior to FES-PET included 24 patients who had CT scans, 23 patients who had bone scans, eight patients who had an MRI, and three patients who had FDG-PET scans.
In addition, biopsy of suspected lesions was performed before FES-PET for four patients and during follow-up for two other cases. PET showed FES-positive metastases in 22 of the 33 patients.
In total, 398 lesions were detected by FES-PET, with 341 lesions located in the bone, 26 in the lymph nodes, 19 in the lung or pleura, eight in the liver, and four in soft tissue.
By comparison, the researchers detected 242 (61%) of the 398 FES-positive lesions on conventional imaging. After comparison between FES-PET and conventional imaging, 79 additional lesions with FES uptake were also detected using conventional techniques. During follow-up, the researchers confirmed 15 more lesions.
 |
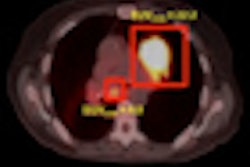
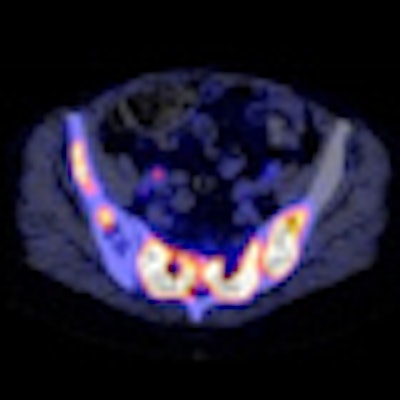
| Bone scan (A), FES-PET (B), and FES-PET/CT (C, D) images of a patient with progressive disease after multiple lines of antihormonal therapy and chemotherapy. Extensive tracer uptake is evident in bone, lymph nodes, and intracerebral regions. FES uptake also appears predominant in bone marrow of this patient, in whom laboratory signs of bone marrow infiltration were present. Images courtesy of the Journal of Nuclear Medicine. |
The results for liver tumors found by FES-PET were not as impressive. A total of 319 metastases were found at conventional imaging. Of that total, FES-PET missed 77 lesions (24%), leading the group to conclude that detecting liver metastases with FES-PET was less than adequate.
"The sensitivity for liver metastases was poor, and quantification of FES uptake in liver lesions was hampered by high physiologic background," van Kruchten and colleagues wrote.
Clinical benefit
Of the 21 patients who underwent FES-PET due to inconclusive workup, 13 were found to have bone lesions, four had lung lesions, three had liver lesions, and one had an abdominal lesion.
Among those 21 patients, 16 had received a bone scan prior to FES-PET, 16 had undergone a CT scan, four had received an MRI, three had received an FDG-PET scan, and three were biopsied. In nine (43%) of the 21 cases, the researchers found elevated uptake of FES at the suspected lesion, confirming the presence of estrogen-receptor-positive metastases.
With the help of FES-PET, referring physicians achieved improved diagnostic understanding for 29 (88%) of the 33 patients, with the modality receiving sole credit for improving that understanding in 18 of 29 cases.
FES-PET also altered therapeutic strategy in 16 (48%) of the 33 patients. In 11 cases, FES-PET was the most important factor in the treatment change, according to the authors.