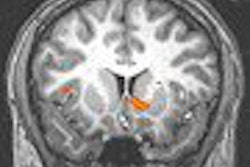
German contrast developer Schering has received marketing approval from the Swedish health authority MPA for its Primovist MR liver imaging agent. The gadolinium-based contrast agent is designed to aid in the detection and characterization of liver lesions, including liver tumors, metastases, as well as other malignant and benign tumors, according to the Berlin-based firm.
Based on a mutual recognition procedure, Schering said it expects to receive European Union registration in most countries within 2004. Submission for approval in Japan and other Asian countries is planned this year, Schering said.
April 5, 2004
Related Reading
Schering gets European OK for Zevalin for NHL, January 22, 2004
Schering starts testing optical mammo, September 12, 2003
Currency effects weigh down Schering results, August 8, 2003
Epix, Schering to partner on MRI contrast, May 27, 2003
Schering diagnostics business dips in 2002, February 28, 2003
Copyright © 2004 AuntMinnie.com