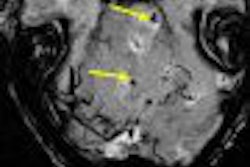
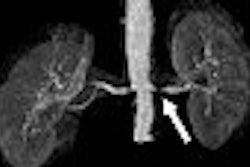
MRI contrast developer Epix Pharmaceuticals of Lexington, MA, has reached an agreement with the U.S. Food and Drug Administration (FDA) on a proposal to reread images collected in trials of its blood-pool MR angiography agent, Vasovist, the company reported.
Epix has received a letter from the FDA that confirms that the protocol design and statistical analysis agreed on by the agency and Epix adequately address objectives necessary to resubmit a new drug application (NDA) for Vasovist.
The company has initiated the rereading of images obtained in prior phase III studies, and expects to submit those results in mid-2008. Vasovist has been approved for marketing in 33 countries outside of the U.S., Epix said.
Related Reading
Epix to raise $16.3 million, November 12, 2007
Epix receives FDA response, June 19, 2007
Epix posts big Q1 loss, May 9, 2007
Epix chairman to retire, May 2, 2007
Epix regains Nasdaq compliance, April 13, 2007
Copyright © 2008 AuntMinnie.com