(Booth 4751) Mednovus comes to RSNA 2008 with its Sentinel GS 2.0 line of ferromagnetic detection portals, designed to prevent the introduction of metal devices into MRI suites.
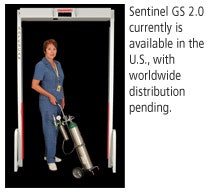
GS technology provides an average of four times greater sensitivity than Mednovus' own fringe-field-dependent or Earth's-field-dependent (non-GS) portals, according to the company.
Its intended use is for superficial pre-entry screening for MRI suites. It currently is not approved for identifying ferromagnetic threats internal to the body of the subject. That feature currently is in development, funded by a grant from the National Institutes of Health.