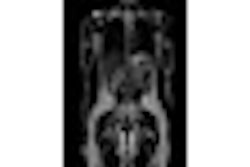
Magmedix of Fitchburg, MA, plans to showcase developments with its Accusorb MRI radiofrequency (RF) shielding material at the 2008 RSNA meeting.
Accusorb is designed to isolate areas of the body from RF deposition and eliminate artifacts resulting from excitation of anatomical areas adjacent to targeted locations.
The U.S. Food and Drug Administration (FDA) has cleared the device as an MRI isolation blanket, class I device. Magmedix introduced Accusorb at RSNA 2006.
Accusorb has lowered actual scan sequence times by as much as 70%, resulting in as many as three additional scans per month per scanner, according to Magmedix.
Related Reading
Road to RSNA, MRI, Magmedix, October 31, 2008
Copyright © 2008 AuntMinnie.com