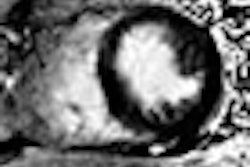
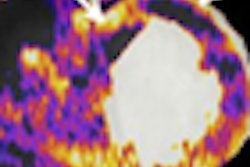
NordicNeuroLab of Bergen, Norway, announced that its nordicICE BOLD and DTI Modules used in functional MRI (fMRI) studies have received U.S. Food and Drug Administration 510(k) clearance.
In the latest version of nordicICE, the BOLD and DTI Wizard simplifies and optimizes workflow associated with analysis of diffusion tensor imaging and BOLD fMRI data. The wizard features a step-by-step interface that guides the user through the process of loading, analyzing, and visualizing multimodality imaging data.
A Windows-based application offers utility in clinical as well as research settings, according to the company.
Related Reading
Road to RSNA, Advanced Visualization, NordicNeuroLab, November 6, 2008
NordicImagingLab releases nordicice 2.3, July 21, 2008
NordicNeuroLab nets 510(k) for new software, June 3, 2008
NordicNeuroLab forms U.S. office, April 16, 2008
NordicNeuroLab wins clearance for fMRI system, January 4, 2008
Copyright © 2008 AuntMinnie.com