Ascelia Pharma is highlighting results from a phase III study for its liver imaging drug candidate, Orviglance.
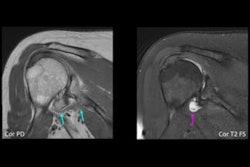
The study showed superior performance for visualizing focal liver lesions with Orviglance compared with unenhanced MRI, with statistical significance for all study three readers (< 0.001). This data marks the completion of Orviglance clinical development with nine studies in 286 patients and healthy volunteers, the company said.
Ascelia Pharma is developing the drug as a contrast agent for use in liver MRI in patients with severely impaired kidney function; Orviglance has been granted orphan drug designation from the U.S. Food and Drug Administration (FDA). The company plans to submit a new drug application (NDA) to the FDA for Orviglance by mid-2025, it said.