Medical isotope producer Advanced Medical Isotope (AMIC) has submitted data on its RadioGel device to the U.S. Food and Drug Administration (FDA) and requested a collaborative meeting.
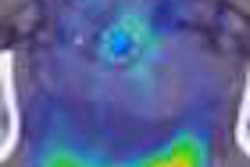
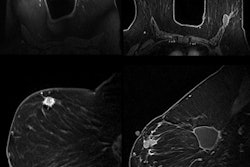
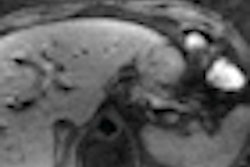
The injectable RadioGel products deliver the insoluble radioisotope yttrium-90 (Y-90) to specific areas to treat cancerous tumors that cannot be surgically removed. The treatment is designed to minimize exposure to surrounding healthy tissues and organs.
According to AMIC, RadioGel has shown favorable results in animal studies recently concluded at the Pacific Northwest National Laboratory.
The FDA submission is the first step in the premarket review process. AMIC is also submitting information to the Washington State Department of Health that is required for a sealed source and device (SS&D) registration, which would allow the use of RadioGel in hospitals and clinics.
AMIC plans to manufacture RadioGel in Washington state and to build a new facility in Richland, WA, which will include a new cyclotron to produce enough technetium-99m to meet the clinical needs of large urban centers.