Imaging biomarkers show promise as tools to assess patient response to chemotherapy and radiation therapy. Research on the use of biomarkers for brain tumors and esophageal cancers was presented in scientific sessions at the Cancer Imaging and Radiation Therapy Symposium, held February 8 and 9 in Orlando, FL.
CT texture analysis
Treatment for early-stage esophageal cancer frequently includes curative surgery. Because surgical outcomes tend to be poor, neoadjuvant chemotherapy is usually prescribed to improve the odds of preventing local or distant cancer recurrence.
At present, there is no established imaging or histological biomarker that identifies responders or good prognosis in patients who would benefit from surgery, according to Dr. Connie Yip, an associate consultant in radiation oncology at the National Cancer Centre in Singapore and a clinical research fellow at King's College London.
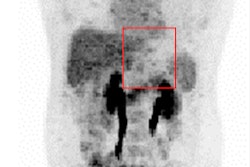
However, CT texture analysis of primary tumors may be a potential imaging biomarker in localized esophageal cancer following neoadjuvant chemotherapy, Yip told attendees at the conference, which was cosponsored by the American Society for Radiation Oncology (ASTRO) and RSNA.
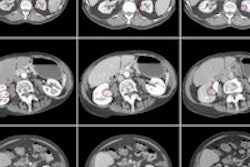
Yip and colleagues evaluated tumoral texture analyses on CT scans that were performed before and after the use of chemotherapy in 31 patients with localized resectable esophageal cancer. The patients were treated in London at Guy's and St. Thomas' NHS Foundation Trust and King's College Hospital NHS Foundation Trust.
All patients had received neoadjuvant platinum and fluorouracil-based chemotherapy, followed by surgery, between 2007 and 2010. Eleven patients received adjuvant treatment.
The majority of the patient cohort had been diagnosed with either stage II (12 patients) or stage III (17 patients) disease. The group ranged in age from 49 to 77 years, with a median age of 63 years.
The researchers used proprietary software (TexRAD, University of Sussex) to perform CT texture analysis of the primary tumor on staging and postchemotherapy scans. Texture parameters included mean gray-level intensity (MGI), entropy, uniformity, skewness, and standard deviation of histogram. Each of these parameters was derived for four filter values to highlight structures of different spatial width.
A subgroup of 26 patients was established as responders and nonresponders based on pathological Mandard score. Responders had a score of 1 to 3, while nonresponders had a score of 4 to 5. All patients were followed for a median of 21.9 months.
"Primary tumors became more homogenous following chemotherapy, as entropy decreased and uniformity increased," reported Yip. "A smaller change in skewness following chemotherapy was a significant prognostic factor, and median overall survival was 36.1 months compared to 11.1 months."
Lower baseline entropy and lower post-treatment MGI were also associated with improved survival, although they demonstrated only a trend toward significance.
"Though these results are for a very small number of patients, they suggest that the tumoral texture features may provide valuable information that could help us to distinguish which patients will do well following chemotherapy and which ones will do poorly," she said. "As a biomarker for treatment efficacy, this technique could save patients from unnecessary surgery and provide more definitive guidance in developing patient treatment plans with improved outcomes."
Diffusion abnormality index
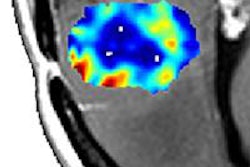
A diffusion abnormality index (DAI) shows promise as an imaging biomarker to measure brain tumor response to radiation therapy, according to another study presented this week, this one from the University of Michigan in Ann Arbor.
The study included 20 patients who had brain metastases and were treated with whole-brain radiotherapy. Lead author Reza Farjam, a PhD candidate in biomedical engineering and radiation oncology, and colleagues categorized the 45 brain lesions found in the patient cohort. These included 16 responsive lesions, 18 stable lesions, and 11 progressive lesions.
Diffusion measurements were taken prior to radiotherapy, two weeks after the start of treatment, and one month after treatment had been completed. For each patient, a normal tissue apparent diffusion coefficient (ADC) histogram was used to divide the tumor ADC histogram into three regions: low (high cellularity), normal, and high (edema and necrosis) diffusion.
From analyzing the complex behavior in ADC of brain metastases from preradiation therapy to two weeks after starting treatment, the investigators developed a new diffusion index. This DAI included both high and low ADC contributions for prediction of post-treatment tumor response.
Sensitivity and specificity of the change in DAI from baseline to the end of therapy were compared with the changes in gross tumor volume for the same time period. The changes were valuable in predicting nonresponsive lesions post-treatment.
"Early prediction of brain tumor response to radiation therapy is vital in providing the most appropriate radiation doses to each lesion," Farjam said. "The data from our small study demonstrated that DAI may be a good biomarker to predict brain tumor response. Further study is needed to improve early prediction of tumor response to radiation therapy and to help us provide patients who are receiving treatment for brain cancer with more accurate information about their treatment progress."