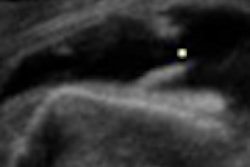
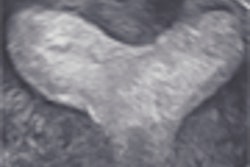
Medical imaging developer Eigen has received U.S. Food and Drug Administration (FDA) clearance for its ei-Nav/Artemis prostate cancer imaging device.
Artemis employs 3D/4D imaging technology to enhance urologists' existing ultrasound machines, according to the Grass Valley, CA-based vendor. This allows urologists to virtually see inside the prostate in real-time, guide 4D needle navigation, map biopsy locations, and generate an image of 3D biopsy coordinates for future reference, Eigen said.
The first Artemis systems will soon be in use at the University of Colorado Health Sciences Center in Denver and the University of California, San Francisco. The systems are available for immediate order in the U.S, Eigen said.
Related Reading
Road to RSNA, Digital X-Ray, Eigen, October 30, 2006
Eigen taps Stentor vet as CTO, July 27, 2005
Copyright © 2008 AuntMinnie.com