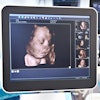
The U.S. Food and Drug Administration (FDA) has cleared Clarius Mobile Health's Clarius OB AI, an algorithm that performs fetal biometry measurements to estimate fetal age, weight, and growth intervals.
 Clarius OB AI automatically highlights key fetal anatomy.Image courtesy of Clarius
Clarius OB AI automatically highlights key fetal anatomy.Image courtesy of Clarius
Clarius OB AI is available now in the U.S. and Canada with the company's Clarius C3 HD3 wireless handheld ultrasound scanner, it said. The deep-learning model was developed using more than 30,000 fetal ultrasound images and enables new ultrasound users, including midwives and nurses, to perform obstetrical (OB) prenatal monitoring, Clarius noted.
Clarius OB AI is the eighth AI model developed in-house by the company for use with its ultrasound scanners, it added.