Dear AuntMinnie Member,
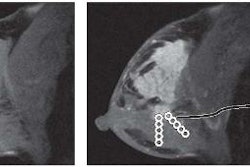
The U.S. Food and Drug Administration (FDA) is planning to relax its regulatory requirements for full-field digital mammography (FFDM) systems in a move that could make it easier for new FFDM technologies to get to market.
In an article in our Women's Imaging Digital Community, staff writer Cynthia Keen describes how the FDA's proposal would eliminate the requirement that new FFDM systems go through the premarket approval (PMA) process before they can be sold in the U.S. The PMA process is more cumbersome and requires clinical trials, unlike the 510(k) process used for most radiology devices.
Vendors are applauding the move, saying that it would enable companies to commercialize new FFDM technologies much more quickly -- perhaps in as few as 90 days. Find out how it could affect breast imaging providers by clicking here, or go to the Women's Imaging Digital Community at women.auntminnie.com.