Women's imaging vendor Hologic is taking a measured approach to commercializing its digital breast tomosynthesis (DBT) technology, with the company starting to take orders for a limited number of the mammography systems from European customers.
But sales in the U.S. are another matter. The Bedford, MA, firm is navigating a tricky course as it endeavors to manage multiple international markets with varying regulatory requirements, all the while trying to preserve sales of its existing conventional full-field digital mammography (FFDM) systems.
 |
| Selenia Dimensions features a gantry modified for tomosynthesis. All images courtesy of Hologic. |
But Hologic won't be implementing a full-bore commercial rollout, at least not initially. The company plans to limit the first batch of shipments to 10 "strategic partners" in Europe, and even these units won't be delivered until September or October.
Differing regulatory standards between the U.S. and Europe for medical devices are complicating the company's rollout plans. In the U.S., firms with DBT systems are being required to go through the U.S. Food and Drug Administration's premarket approval (PMA) process, which requires clinical trials and an extensive analysis of regulatory applications by the FDA's team.
In Europe, the situation for this class of medical devices is somewhat different. In addition to receiving a CE Mark, Hologic is working to address country-specific requirements that will have to be met in order to market and sell the product.
Hologic submitted its original PMA for Selenia Dimensions last year, and has responded to FDA requests for additional data, Cascella said. The company isn't sure whether the FDA will hold a panel hearing as part of the review process for the product, but the firm does discuss the application with the agency every several weeks, he said.
The company is hoping to receive FDA approval by the end of 2008, and make a major commercial launch of Selenia Dimensions at this year's RSNA show.
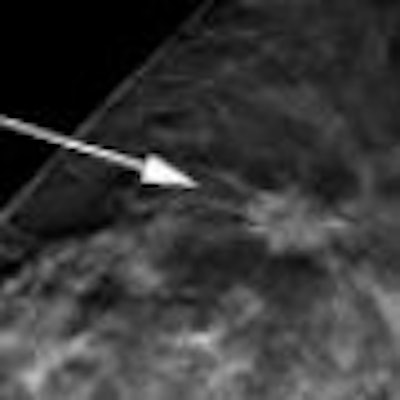
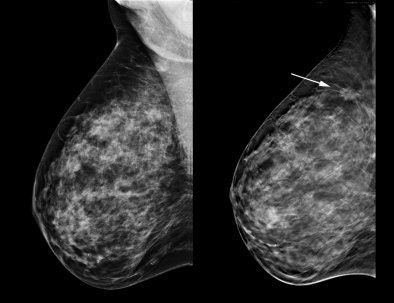 |
| Tomosynthesis advocates believe the technology can help visualize structures that might be obscured by overlapping tissue on 2D mammography studies. |
European customers who buy a Selenia Dimensions system now will get what the company calls a "tomo-ready" product -- a 2D FFDM system that features the motorized gantry required to collect the multiple image slices of the breast that are then reconstructed into a tomosynthesis image. The system will also feature other electromechanical "hooks" required to perform tomosynthesis, as well as a more advanced acquisition console. Cascella estimates that this system will cost 10% to 15% more than a standard Selenia unit that doesn't support tomo.
When tomosynthesis becomes available, the 2D Dimensions unit can then be upgraded to 3D tomosynthesis through a software upgrade. Hologic hasn't set an exact list price for the upgrade, but it could be somewhere in the range of $100,000 to $200,000.
Another wrinkle is the reimbursement situation -- currently, 3D digital mammography has no reimbursement code. This is sure to be an issue in an environment as sensitive to reimbursement as mammography, where many U.S. breast centers conduct screening mammography at a loss due to low reimbursement levels.
But Hologic is taking the long view on DBT, and is predicting that it may not be until 2010 that the technology really comes into its own, as reimbursement levels are established.
"We are talking about a product that has the ability to change the way we do screening mammography, but it needs to get reviewed and vetted by clinicians," Cascella said. "We've done a lot of work with radiologists, but until it's in the field in a much broader way, we'll learn a lot and we'll make modifications to the product as we learn."
By Brian Casey
AuntMinnie.com staff writer
March 26, 2008
Related Reading
ECR sessions examine DBT's effectiveness, March 7, 2008
Hologic readies stock split, January 30, 2008
Hologic files suit against SenoRx, January 11, 2008
Hologic joins Nasdaq-100, December 20, 2007
Hologic to issue $1.5 billion note, December 6, 2007
Copyright © 2008 AuntMinnie.com



















