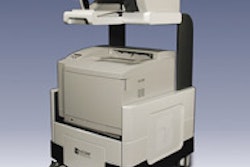
Breast cancer detection developer Biofield of Atlanta is making progress in bringing its Breast Cancer Diagnostic System (BDS) to the U.S. market. The Atlanta company has secured a recommendation from a third-party review agency, with the next step a submission to the Food and Drug Administration.
Biofield received a recommendation from KEMA Registered Quality, an FDA-sanctioned third-party reviewer, that BDS may be used as an adjunct to support other modalities such as mammography, clinical breast exam, sonography, and MRI for breast cancer diagnosis, according to the firm.
BDS measures electrical activity in the breast to detect epithelial cancers, including breast cancer. The company believes the device can be a useful adjunct in helping to reduce surgical biopsies on suspicious breast lesions.
KEMA's submission to the FDA is the next step in obtaining regulatory clearance that will allow for the marketing and sale of the BDS in the U.S., Biofield said.
By AuntMinnie.com staff writers
November 16, 2004
Related Reading
Biofield nets African distribution deal, December 7, 2001
Biofield to try again with FDA, September 18, 2001
Biofield begins Europe survey, August 31, 2000
Copyright © 2004AuntMinnie.com