Endoscopic ultrasound-guided biopsy technology developer Limaca Medical said that it has raised nearly $1.3 million of a $1.5 million funding round.
Limaca said that the funds are being used to support first-in-human procedures, postmarket clinical studies, and obtaining regulatory approvals for its Precision endoscopic ultrasound-guided biopsy device for gastrointestinal and other solid tumor cancers. Precision features an automated revolving needle for acquiring core tissue for histopathology and advanced genetic profiling, the firm said.
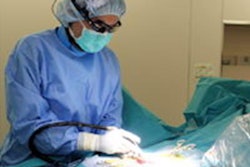
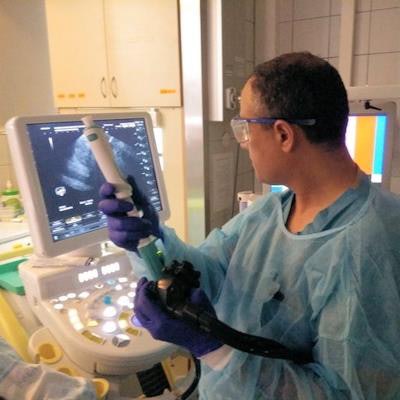
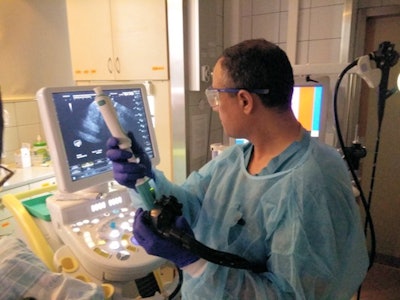 Dr. Iyad Khamaysi of Rambam Health Care Campus in Haifa, Israel, performing the first in-human study utilizing Limaca's Precision endoscopic ultrasound-guided biopsy device. Image courtesy of Limaca.
Dr. Iyad Khamaysi of Rambam Health Care Campus in Haifa, Israel, performing the first in-human study utilizing Limaca's Precision endoscopic ultrasound-guided biopsy device. Image courtesy of Limaca.So far, 10 patients have been enrolled in Limaca's first-in-human study at Rambam Health Care Campus in Israel. The study is comparing Precision to standard-of-care endoscopic ultrasound biopsy devices; preliminary results have shown ease of use, safety, and improved sample capture, according to Limaca.
The financing round included participation from Trendelines, Agriline; Limaca Chairman Carl Rickenbaugh; and a private investor, Limaca said.