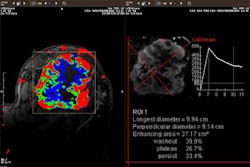
R2 Technology's pre-market approval (PMA) application for its ImageChecker CT computer-aided detection system has been accepted for review by the Food and Drug Administration. The Sunnyvale, CA-based firm filed the PMA on April 16 for the system, which is designed for the detection of lung nodules in multi-detector CT images. ImageChecker CT was introduced commercially in Europe in March.
By AuntMinnie.com staff writers
June 25, 2003
Related Reading
R2 adds North American ImageChecker CT installs, May 6, 2003
R2 outfits Baptist Health South Florida, April 17, 2003
R2, HPG sign deal, April 8, 2003
R2 inks PACS/CAD deal with Sectra, March 9, 2003
R2 secures first beta site for ImageChecker CT, March 7, 2003
Copyright © 2003 AuntMinnie.com