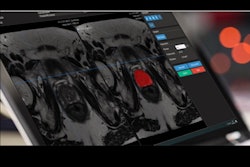
Artificial intelligence (AI) software developer Quantib has received U.S. Food and Drug Administration (FDA) clearance for its AI workflow integration platform.
With the platform, radiologists can integrate Quantib's current and future AI algorithms into their daily workflow, according to the vendor. The FDA clearance is the fifth for the Rotterdam, Netherlands-based firm.
Quantib also noted that it's preparing to open an office in New York -- its first office in the U.S.