CNS Neoplasms:
Primary CNS neoplasms are uncommon (7 to 19 cases per 100,000 people) [9]. Brain metastases are up to 10 times more common than primary brain tumors and occur in 20-40% of patients with cancer [9]. The most common tumor types to metastasize to the brain are lung, breast, and melanoma [29].
Primary CNS gliomas are of 3 main types- astrocytomas, oligodendrogliomas (more likely to contain calcification and have a better overall prognosis), and mixed oligoastrocytomas [29]. The tumors are further classified as low grade (WHO grades I and II) or high grade (WHO grades III and IV) based upon the degree of nuclear atypia, mitoses, microvascular proliferation, and necrosis [29]. There are 3-subtypes of low grade gliomas- pilocystic astrocytoma (grade I), astrocytoma (grade II), and oligodendroglioma (grade II) [29]. High grade gliomas include anaplastic tumors (grade III) and glioblastoma (grade IV) [29]. GLioblastoma is the most malignant and most common glioma- accounting for 45-50% of cases [29]. The mean age of onset for glioblastoma is 61 years, and 40 years for anaplastic astrocytoma [29]. The clinical course of glioblastoma is usually rapid and fatal with a median survival or 1 year [29]. The median survival for anaplastic tumors in 2-3 years [29]. Low-grade tumors are more commonly seen in younger patients [29].
Treatment for grade III and IV tumors is surgical resection to the most feasible extent, followed by adjuvant radiotherapy to improve survival [29]. Concurrent chemotherapy with temozolomide has also been shown to improve survival in patients with glioblastomas [29]. Low grade gliomas are more indolent, but can still be fatal lesions [29]. For pilocytic astrocytomas, surgical resection alone leads to a cure or long-term survival (20 year survival of 80%) [29]. Treatment for other low grade gliomas is more controversial and can include surgical resection of the full extent of the tumor and adjuvant radiotherapy [29].
Physiology for PET imaging of CNS malignancies:
A predominant biochemical feature of rapidly growing tumor cells is an ability to sustain high rates of glycolysis under anaerobic conditions. Glucose (or FDG) utilization in tumors is characterized by increased aerobic and anaerobic glucose metabolism, an increased number of glycolytic enzymes, and increased cellular glucose transport.
One drawback of CNS tumor imaging is that FDG PET has limited ability to distinguish uptake in primary CNS neoplasms from the normally high background gray matter brain activity- especially for small lesions and low grade lesions [11,29] (FDG uptake in low grade tumors is usually similar to normal white matter and uptake in high grade tumors can be less than or similar to that of normal gray matter [29]). Glucose loading may help to improve lesion detection by causing a more significant suppression of FDG uptake in normal brain tissue compared to neoplastic lesions [29]. This failure to suppress uptake is most likely due to a lack of the normal physiologic control of glucose metabolism in neoplastic lesions [1]. Coregistration with MR or CT images has been shown to greatly aid in lesion detection and the PET exam should always be interpreted in conjunction with an anatomic imaging study [11,29]. In many cases, increasing the time between FDG injection and the time of scanning (i.e.- delayed imaging out to 3-8 hours) will also result in greater lesion conspicuity [16,29].
Primary lesion:
PET FDG examination:
Adults receive 10 mCi of FDG injected IV in a room with low ambient light and noise. Following injection patients rest quietly with their eyes closed until imaging between 30-60 minutes after injection. Scans can be corrected for attenuation using an emission scan or with empiric uniform correction [9]. Correlation with anatomic imaging studies is essential for accurate exam interpretation. The amount of intracellular FDG is proportionate to the rate of glucose transport and intracellular phosphorylation [9]. Malignant cells generally have high rates of aerobic glucose metabolism [9]. FDG uptake within an astrocytoma has good correlation with the histologic grade of the tumor [11]. Poorly differentiated high grade tumors (Grades III and IV) commonly exhibit increased FDG uptake similar to gray matter, while uptake is typically only mild in low grade (Grade I-II) lesions (similar to white matter activity) [11]. A few low grade tumors, such as pilocystic astrocytoma and pituitary adenoma may have high FDG uptake [9]. Some metastases may also have metabolic activity less than normal cortex [9]. Higher grade malignant lesions may also show low FDG accumulation if they have an increased reliance on anaerobic metabolism [9].
FDG imaging may also provide prognostic information. In patients with high grade lesions, those with normal or hypometabolic activity on FDG imaging had an improved one year survival (75%) compared to patients with hypermetabolic lesions (29% one year survival) [2,11].
Brain tumors are often comprised of heterogeneous cellular elements [11]. FDG PET imaging can aid in localizing the most hypermetabolic region of a lesion for sterotactic biopsy [11]. Additionally, PET imaging provides additional information which can be incorporated into radiation planning for optimization of patient therapy [14].
Other agents used for CNS malignancy imaging:
Amino acid tracers- amino acid tracers are more sensitive than 18F-FDG in imaging tumors due to their low uptake in normal brain tissue that results in enhaced contrast between the tumor and adjacent normal parenchyma [29,31]. Amino acids are transported into cells via carrier mediated processes and increased amino acid transport seen in tumor cells involves all phases of the cell cycle [29].
11C-L-methylmethionine (measuring amino acid uptake/protein synthesis) also demonstrates increased uptake in 80 to 90% of malignant brain tumors (tumor cells require an external supply of methionine). Uptake of the tracer seems to correlate with histologic tumor grade, with increased activity in higher grade lesions (grade III and IV), but only mild uptake in grade II tumors. Because of low uptake in the normal cortex, C-11 methionine imaging appears to provide the greatest lesion-to-background ratios (all tumors have a ratio of much greater than 1:1). Reported sensitivity can be as high as 97% for high grade lesions, but sensitivity is much lower for low grade tumors [10]. The main limitation of 11C-L-methylmethionine is the short physical half-life of the agent which prevents its use only at centers with an on-site cyclotron [19]. Unfortunately, uptake can also be seen in brain abscesses [23].
18F-FLT (fluorothymidine) measures thymidine kinase-1 activity which is a reflection of tumor proliferation [17]. 18F-FLT is not taken up in normal brain cells due to essentially no proliferative activity and and intact blood-brain barrier [31]. Uptake in CNS neoplasms is rapid with peak activity at 5-10 minutes after injection [17]. Activity remains stable up to 75 minutes which permits PET imaging [17]. Because there is little uptake of 18F-FLT in the normal brain which makes lesions more conspicuous compared to FDG imaging which suffers from high basal CNS uptake [17]. 18F-FLT uptake is seen in the cranial bone marrow and venous sinuses [20]. As with FDG, 18F-FLT uptake correlates with tumor grade- with higher uptake in grade III and IV lesions and little appreciable uptake in lower grade lesions [17,25]. Non-contrast enhancing lesions with intact blood brain barriers also do not demonstrate significant FLT accumulation [25]. Patients with 18F-FLT positive lesions have a worse prognosis compared to patients with negative scans [17]. The sensitivity of 18F-FLT for the detection of CNS tumors (about 78%) is lower than for 11C-L-methylmethionine (about 91%) - especially for low grade astrocytoms [20]. Data regarding the use of 18F-FLT in the evaluation of tumor versus radiation necrosis is not available [17].
18F-fluoroethyl-L-tyrosine (18F-FET or 18F-FYR) is an agent that can assess protein synthesis within malignant lesions. This amino acid analog is taken up via the transport system L, but is not actually incorporated into proteins [21]. The agent is able to identify both low and high grade gliomas [21,23] and results are similar to those with 11C-MET [26]. In low grade (grade II) gliomas, between 66-82% of patients will demonstrate increased tracer uptake compared to normal brain tissue [26,28]. FET uptake also correlates with prognosis- the most favorable prognosis is associated with FET uptake that is the same or less than that of the surrounding brain tissue [26]. Once a low-grade glioma exhibits increased FET uptake, there seems to be a change in the biologic behavior that is associated with a worse prognosis [26]. Although the agent was previously felt to be able to reliably distinguish tumor from non-tumorous lesions, false positive findings have been described in patients with brain abscesses and demyelinating disease [23]. The agent is also taken up in meningiomas [27].
18F-FDOPA is an agent normally used for imaging of neuroendocrine tumors, however, excellent uptake in CNS neoplasms also occurs [24,29]. The uptake mechanism is not yet established but is likely mediated via a specific transport system, rather than breakdown of the BBB [24,29]. The agent does not appear to be trapped within the tumors as tracer washout can be seen over time- the washout is more rapid from high grade tumors and slower from low grade lesions [30]. Uptake appears to be similar for both low grade and high grade CNS lesions [24,29]. Although measured tumor SUV for 18F-FDOPA is lower than for FDG, 18F-FDOPA is more accurate than FDG imaging of CNS neoplasms due to the low uptake in normal brain tissue which improves lesion conspicuity [24]. Overall sensitivity for the evaluation or primary or recurrent CNS neoplasm has been report to be 96%, specificity 43%, accuracy 83%, PPV 85%, and a NPV 0f 75% [24]. Another benefit is that tumor uptake appears to peak between 10-30 minutes following injection which permits early imaging [29].
18F-fluoromisonidazole (18F-FMISO) is a PET tracer used for the evaluation of tissue hypoxia (metabolites of the agent are trapped exclusively in hypoxic cells [29]) [22]. Tumors with greater degrees of hypoxia have been shown to be more resistant to radiation and chemotherapy [22]. Greater 18F-FMISO uptake is generally observed in high grade gliomas compared to low grade lesions [22]. 18F-FMISO glioma uptake has been associated with a decreased response to therapy and a worse prognosis [22].
Blood flow imaging in CNS neoplasms:
On cerebral blood flow imaging, gliomas and cerebral metastases generally have variable CBF which is often mildly reduced in comparison to normal brain tissue. CBF and CBV within the lesion have not been shown to correlate with the tumor grade. Oxygen extraction and oxidative metabolism are usually markedly reduced in brain tumors, despite the heightened glucose utilization.
A drawback of PET imaging is that uptake of both FDG (typically spotty) and 11C-methionine (more prominent) has been described surrounding brain hematomas on scans performed between 20 and 30 days following the event, possibly secondary to a subacute gliotic reaction about the hemorrhage. This activity will decrease and disappear over time (2 to 3 months), but initial differentiation from a neoplasm that has bled may be difficult (between 2-14% of spontaneous intracranial hemorrhage is secondary to tumors). Correlation with the patients contrast enhanced CT or MRI may help as C-11 methionine uptake in non-neoplastic bleeds will be concordant with the contrast enhancement identified about the abnormality. Increased tracer accumulation extending beyond the enhanced area on CT/MRI is indicative of a hemorrhage associated with an underlying CNS neoplasm [3,4]. C-11 methionine uptake has also been reported in brain abscesses [5] and sites of radiation necrosis [6].
Lymphoma versus toxoplasmosis in HIV:
FDG PET has also been used to differentiate lymphoma from toxoplasmosis in patients with AIDS [8]. CNS lymphoma typically demonstrates significantly greater FDG accummulation [18].
Differentiation of recurrent tumor from radiation necrosis:
The general approach to treatment of brain neoplasms is surgical resection of solitary lesions or limited disease, followed by radiation therapy (with or without chemotherapy) [9]. Solitary lesions may alternatively be treated with local field radiotherapy or stereotactic radiosurgery, while multiple or metastatic lesions receive whole-brain radiation [9]. Anatomic alterations and scarring after therapy can impair proper identification of residual or recurrent neoplasm on conventional imaging studies [13].
Radiation injury occurs in 5-37% of cases [9]. Radiation injury to the brain is the major dose-limiting complication of radiotherapy [9]. Although the term "radiation necrosis" is used to describe radiation injury, it is inaccurate because pathologically radiation injury is not limited to necrosis [9]. The incidence of radiation injury depends on the total dose and rate of delivery (fractionalization) [9]. Very young children are more susceptible than adults and chemotherapy (adriamycin and methotrexate) can potentiate radiation injury [9].
Radiation injury can be divided into acute, early-delayed, and late-delayed stages [9]. Late-delayed radiation injury occurs in 5-37% of cases, months to years (10 years) following XRT. About 70% of cases occur in the first 2 years after treatment [9]. The primary mechanism of late-delayed radiation injury is vascular endothelial injury or direct damage to oligodendroglia [9]. The white matter is affected more than the gray matter [9]. Edema, mass effect, and blood-brain barrier disruption (which permits contrast enhancement) are commonly associated with this condition and differentiation from recurrent tumor can be difficult on conventional imaging exams [19]. Treatment of radiation injury range from conservative measures to control intracranial pressure to surgical excision of the edematous mass [9].
Following irradiation, gliomas are not homogeneous and even a sterotactic biopsy may be inaccurate- sampling scarred or gliotic areas [13]. PET studies with FDG have shown that recurrent tumor exhibits hypermetabolism of glucose, while non-necrotic irradiated brain shows hypometabolism, and necrotic brain has no detectable metabolic activity [9]. An exam should be considered suggestive of recurrence if lesion activity is above the expected background activity of adjacent brain tissue [29]. False negative FDG PET exams can occur with lesions with a small or microscopic tumor volume [9]. FDG PET may be less sensitive in differentiating recurrence from radiation injury in low grade (well-differentiated) tumors due to their inherently lower metabolic activity [9]. A reversible decrease in metabolic activity in viable tumors in the immediate post radiation period may also result in a decrease in FDG accumulation [9]. Because of the usual high metabolic activity in the cerebellar hemispheres, it can be difficult to detect contrast between brain tumor recurrence and adjacent normal tissue [7]. False-positive scans may be caused by radiation injury, which activates repair mechanisms that can increase aerobic glucose metabolism [9]. PET imaging for recurrence should not be performed sooner than 6 weeks following completion of radiation therapy [29]. Normal healing during the immediate post-surgical period (up to 3 months) may also result in a false-positive exam [9]. Other causes of focal increased glucose metabolism include seizure activity and abscesses [9]. Overall, FDG PET has a sensitivity of 73-86%, and a specificity of 40-94% in the differentiation of recurrent tumor from radiation brain injury [9,13,29]. Diagnostic performance appears to be worse in lesions treated with stereotactic radiosurgery [29]. PET imaging has been shown to be clearly superior to Tc-MIBI for the differentiation of recurrent neoplasm from post-therapy change [13].
18F-FET may prove to be a useful agent for distinguishing radiation necrosis from tumor recurrence [29]. The agent is not accumulated in macrophages- a common inflammatory mediator [29].
|
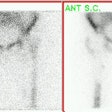
CNS recurrent glioma: The patient below had a history of a right temporal lobe glioma. The lesion had been treated with surgery and radiation. A follow-up MR exam demonstrated areas of enhancement in the right temporal lobe on post-gadolinium images (black arrows). MR spectroscopy was inconclusive. The FDG PET exam demonstrated a hypermetabolic focus in the area of MR signal abnormality consistent with recurrent glioma (white arrows). |
|
|
In the post-treatment setting, FDG PET imaging can provide additional information which can greatly aid in patient management. One added benefit of FDG PET imaging is in selection of the best site for biopsy of a focal lesion [9]. Biopsy can be directed at the most metabolically active site to enhance diagnostic yield. FDG imaging also better defines sites of residual tumor (when compared to MR imaging) which can subsequently be targeted for escalated radiation therapy [12].
|
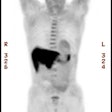
CNS radiation necrosis: The patient below had a history of a solitary brain metastasis from non-small cell lung cancer. The lesion had been resected and the patient had received radiation therapy to the area. A follow-up MR exam revealed a ring enhancing region in the left parietal-temporal area on post-gadolinium enhanced images (white arrows). A FDG PET exam revealed no tracer uptake consistent with post-surgical and post radiation change. |
|
|
REFERENCES:
(1) J Nucl Med 1994; Ishizu K, et al. Effects of hyperglycemia on FDG uptake in human
brain and glioma. 35 (7): 1104-09
(2) Cancer 1988, 62:1074-78
(3) J Nucl Med 1994; Dethy S, et al. Carbon-11-methionine and fluorine-18-FDG PET study in brain hematoma. 35 (7) 1162-66
(4) J Nucl Med 1995; Ogawa T, et al. Carbon-11-methionine PET evaluation of intracerebral hematoma: Distinguishing neoplastic from non-neoplastic hematoma. 36 (12): 2175-79
(5) J Comput Assist Tomogr 1993; Ishii K, et al. High L-methyl-[11C] methionine uptake in brain abscess: A PET study. 17: 660-1 (No abstract available)
(6) Acta Radiol 1991; Ogawa T, et al. Clinical value of PET with 18F-fluorodeoxyglucose and L-methyl-11C-methionine for diagnosis of recurrent brain tumor and radiation injury. 32: 197-202
(7) J Nucl Med 1995; 36 (1): 163
(8) J Nucl Med 1999; Delbeke D. Oncological applications of FDG PET imaging: Brain tumors, colorectal cancer, lymphoma, and melanoma. 40: 591-603
(9) J Nucl Med 2001; Langleben DD, Segall GM. PET in differentiation of recurrent brain tumor from radiation injury. 41: 1861-1867
(10) J Nucl Med 2001; Jager PL, et al. Radiolabeled amino acids: Basic aspects and clinical applications in oncology. 42: 432-445
(11) Radiol Clin N Am 2001; Hagge RJ, et al. Positron emission tomography. Brain tumors and lung cancer. 39: 871-881
(12) J Nucl Med 2002; Tralins KS, et al. Volumetric analysis of 18F-FDG PET in gliobastoma multiforme: prognostic information and possible role in definition of target volumes in radiation dose escalation. 43: 1667-1673
(13) J Nucl Med 2004; Henze M, et al. PET and SPECT for detection of tumor progression in irradiated low-grade astrocytoma: a receiver-operating-characteristics analysis. 45: 579-586
(14) J Nucl Med 2004; Levivier M, et al. Use of stereotactic PET images in dosimetry planning of radiosurgery for brain tumors: clinical experience and proposed classification. 45: 1146-1154
(15) J Nucl Med 2004; Pirotte B, et al. Comparison of 18F-FDG and 11C-methionine for PET-guided sterotactic brain biopsy of gliomas. 45: 1293-1298
(16) J Nucl Med 2004; Spence AM, et al. 18F-FDG PET of gliomas at delayed intervals: improved distinction between tumor and normal gray matter. 45: 1653-1659
(17) J Nucl Med 2005; Chen W, et al. Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. 46: 945-952
(18) J Nucl Med 2005; Love C, et al. FDG PET of infection and inflammation. 25: 1357-1368
(19) Radiol Clin N Am 2005; Hustinx R, et al. PET imaging for differentiating recurrent brain tumor from radiation necrosis. 43: 35-47
(20) J Nucl Med 2005; Jacobs AH, et al. 18F-fluoro-L-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. 46: 1948-1958
(21) J Nucl Med 2006; Popperl G, et al. Analysis of 18F-FET PET for grading of recurrent gliomas: is evaluation of uptake kinetics superior to standard methods. 47: 393-403
(22) J Nucl Med 2006; Cher LM, et al. Correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in gliomas using 18F-fluoromisonidazole, 18F-FDG PET, and immunohistochemical studies. 47: 410-418
(23) J Nucl Med 2006; Floeth FW, et al. 18F-FET PET differentiation of ring-enhancing brain lesions. 47: 776-782
(24) J Nucl Med 2006; Chen W, et al. 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. 47: 904-911
(25) J Nucl Med 2006; Muzi M, et al. Kinetic analysis of 3'-deoxy-3'-18F-fluorothymidine in patients with gliomas. 47: 1612-1621
(26) J Nucl Med 2007; Floeth FW, et al. Prognostic value of O-(2-18F-fluoroethyl)-L-tyrosine PET and MRI in low-grade glioma. 48: 519-527
(27) J Nucl Med 2007; Rutten I, et al. PET/CT of skull base meningiomas using 2-18F-fluoro-L-tyrosine: initial report, 48: 720-725
(28) J Nucl Med 2007; Wyss MT, et al. Spatial heterogeneity of low-grade gliomas at the capillary level: a PET study on tumor blood flow and amino acid uptake. 48: 1047-1052
(29) J Nucl Med 2007; Chen W. Clinical applications of PET in brain tumors. 48: 1468-1481
(30) J Nucl Med 2007; Schiepers C, et al. 18F-FDOPA kinetics in brain tumors. 48: 1651-1661
(31) Radiology 2007; Hammoud DA, et al. Molecular neuroimaging: from conventional to emerging technologies. 245: 21-42