Lymphoma:
Background:
There are 55,000 to 60,000 new cases of non-Hodgkin’s lymphoma (NHL) each year in the U.S. and 24,000 deaths [12]. The incidence of NHL has been increasing approximately 3%-5% per year for the last three decades and the incidence is now twice as high as that of Hodgkin's lymphoma [12,36,43]. Hodgkin’s disease is much less common than NHL with about 7,500 new cases each year in the United States [12]. In men aged 15-54 years of age, Hodgkin’s disease is the third leading cause of death, and NHL is the second leading cause of death [2]. Both Hodgkin’s and NHL are amenable to curative therapy and many of the affected patients are young with otherwise good life expectancies. Prognosis, treatment, and survival depend on histologic grade, clinical stage, and response to treatment [36]. FDG PET imaging can play a significant role in the staging and management of patients with lymphoma. A meta-analysis showed a median sensitivity of 90.3% and a median specificity of 91.1% for lymphoma staging with dedicated PET [49].
Staging lymphoma:
The Ann Arbor classification which divides disease in 4 stages can be applied to both HD and NHL [35,50,64]. Stage I is disease limited to a single lymph node or lymph node group, above or below the diaphragm, or is extranodal, but has not spread to other organs or lymph nodes; Stage II is disease in 2 or more lymph node groups on the same side of the diaphragm, or one extranodal organ with spread to one or more lymph node groups on the same side of the diaphragm; Stage III is disease in two or more lymph node groups on both sides of the diaphragm; and Stage IV is extensive disease in extranodal sites such as the lung, liver, bone or bone marrow, or other site [35]. If there is local extra-nodal extension a subscript designator "E" is applied [35]. The presence of systemic symptoms (fevers to 38° C or higher, night sweats, or greater than 10% weight loss over 6 months) is designated by the subscript "B" (the absence of systemic symptoms is designated "A") [35]. Bulky disease with nodes larger than 10 cm is designated by the subscript "x" [35].
Non-Hodgkin's Lymphoma:
Non-Hodgkin's lymphomas (NHL) represent a heterogeneous group of lymphoreticular malignancies that differ with regard to histopathology, clinical behavioir, response to therapy, and clinical outcome [25]. Infectious agents have been linked to lymphoma development including viral agents such as the human immunodeficiency virus, T-cell lymphotrophic virus, Ebstein-Barr virus, and bacterial agents such as Helicobacter pylori [64]. NHL's include diffuse large B-cell (most common subtype of non-Hodgkin lymphoma accounting for 31-50% of cases), follicular (22%), marginal zone or MALT (8%), peripheral T-cell (7-15%), small lymphocyte B-cell (7%), mantle cell (6%), and others (11%) [43,46,50,82]. Treatment for NHL is based on several factors, including tumor grade [12].
NHL is broadly grouped into low-, intermediate-, and high-grade disease subgroups [12]. There is a direct correlation between the degree of FDG uptake and the histologic grade of lymphoma [3]. High-grade tumors demonstrate greater metabolic activity (and greater FDG accumulation) than low grade tumors [4]. In NHL, diffuse large B-cell lymphoma and grade 2 or 3 follicular lymphoma demonstrate the highest FDG metabolism [49]. Low-grade lesions (including mucosa-associated lymphoid tissue [MALT] lesions) may not accumulate sufficient FDG to be visualized [16,19]. Other subtypes of lymphoma that are poorly FDG avid on PET include marginal zone lymphoma and peripheral T-cell lymphoma [40], as well as small lymphocytic lymphoma, anaplastic large T-cell lymphoma, and splenic marginal zone lymphoma [47]. FDG avidity for specific types of lymphomas was reviewed in one study- 97% of diffuse large B-cell lymphomas were FDG avid, 95% of follicular lymphomas, 85% of T-cell lymphomas, 83% of small lymphocytic lymphomas, 55% of extranodal marginal zone lymphoma (however, sensitivity varies with location), and 40% of primary cutaneous anaplastic large T-cell lymphomas [47]. For extranodal marginal zone lymphoma, sensitivity is lower for gastric involvement (39%) than for lung or parotid involvement (sensitivity up to 100%) [49].
Low grade NHL accounts for about 40% of new cases. Follciular lymphoma is the most common form of indolent B cell non-Hodgkin lymphoma [60]. Low-grade lymphomas have an indolent course with repeated, but transient responses to therapy [60]. Follicular lymphoma has been linked to a chromosome 14:18 translocation [64]. Most patients are treated with single-agent chemotherapy or conservative watching and waiting [12]. FDG-PET imaging may have only a limited role in managing patients with low-grade lymphoma [12]. As previously mentioned, low-grade lesions (such as MALT lymphomas) are less metabolically active and therefore they may not accumulate significant amounts of FDG resulting in false negative exams [29]. Additionally, the presence of residual disease after treatment may not be clinically relevant as this type of lymphoma generally has no cure, but is indolent with a survival of 10-12 years [12]. Histologic transformation into an agressive lymphoma occurs in 17% of patients after 5 years, 28% after 10 years, and 37% after 15 years [60]. Transformation can be herald by rapid growth of lymph nodes, an elevated LDH level, or development of systemic symptoms [60]. PET may play a role in evaluation of these patients should transformation to a higher-grade lymphoma occur [12,60].
Intermediate-grade lymphoma accounts for about 40% of new cases and treatment consists of combination chemotherapy and involved-field radiation for patients with bulky disease or early-stage non-bulky disease [12]. The role of FDG-PET imaging in patients with intermediate grade lymphoma would be to demonstrate disease extent and therapy response -- with hopes for improved survival with early modifications in chemotherapeutic regimens.
High-grade NHL accounts for 5%-10% of new cases [12]. Diffuse large B-cell lymphoma (DLBCL) is the most frequent subtype of high-grade non-Hodgkin lymphoma [57]. Untreated, high-grade NHL is associated with a survival measured in weeks. Treatment consists of 6-8 cycles of aggressive combination chemotherapy combining cyclophosphamide, adriamycin, vincristine, and prednisone with rituximab (R-CHOP) or 3-4 cycles of radiotherapy for early-stage non-bulky disease [12,63,70]. With treatment, remission can be achieved in about 60% of patients (other authors quote an event-free two year survival of 75% [63]) [12]. Approximately 25-40% of DLBCL patients experience relapse or progression in the first year [82]. High grade lymphomas are very FDG avid and PET imaging can be used for staging and monitoring response to therapy.
Burkitt lymphoma is a high grade B-cell neoplasm associated with EBV, HIV, and chomosomal translocations that cause overexpression of the C-MYC oncogene [81]. Immunodeficiency associated Burkitt lymphoma accounts for about 30-40% of all AIDS-related lymphomas [81].
T-cell lymphomas account for about 12% of cases of non-Hodgkin lymphoma [51]. T-cell lymphoma has an overall poor prognosis (worse than B-cell lymphomas), with a 5-year survival rate of about 15-30% and frequent extranodal involvement [46,51]. In general, T-cell non-Hodgkin's lymphoma can be divided into peripheral (primary nodal and primary systemic [extranodal]) and leukemic subtypes [46]. Most T-cell lymphomas are FDG positive, but indolent lesions will show lower FDG uptake [46]. Systemic anaplastic large cell lymphoma seems to be the most FDG-avid variant of T-cell lymphoma [51]. Below is a description of some of the forms of T-cell lymphoma (TCL):
1. Nodal subtypes:
Peripheral TCL, unspecified: This is the most common subtype of TCL accounting for 60-70% of cases [46]. Generalized lymphadenopathy is the most common finding [46]. About 60% of patients have stage IV disease at the time of diagnosis [51]. A bulky tumor (10 cm in diameter) and extranodal disease are associated with a poor prognosis [46]. Almost all lesions show high FDG accumulation [46].
Angioimmunoblastic TCL: Accounts for 15-20% of cases of TCL and patients typically present with advanced disease (overall median survival is 15 months) [46]. Nodal involvement is associated with organomegaly (hepatospenomegaly), B-symptoms, rash, puritis, pleural effusion, arthritis, and eosinophila [46,51]. Many patients show hypergammaglobulinemia, Coombs positive hemolytic anemia, and evidence of immunodeficiency [51]. The sensitivity of FDG PET for the detection of extracutaneous lesions approaches 78-100% [46,51].
2. Extranodal subtypes:
Cutaneous TCL:
Cutaneous TCL consists of two clinical entities- mycosis fungoides (typically indolent) and Sezary syndrome (a leukemic variant of mycosis fungoides in which characteristic Sezary cells are found in the peripheral blood) [46,51]. Mycosis fungoides is attributed to the clonal expansion of epidermotropic CD4 helper T-cells [51]. The skin lesions appear as flat erythematous patches that evolve into palpable plaques with well-defined edges [46]. Skin lesions can be seen on PET imaging [46]- in one study up to 75% of the lesions associated with mycosis fungoides could be detected [51]. Later in the course of the disease, there is progression to involve lymph nodes and viscera [46]. The 5-year survival decreases from 90% for cutaneous only disease, to almost 0% for disease with visceral involvement [46]. Mycosis fungoides may undergo transformation to a CD30+ anaplastic large cell lymphoma with aggressive behavior in up to 39% of patients [51].
Sezary syndrome is characterized by diffuse erythroderma and can be difficult to detect on PET imaging due to the diffuse nature of the disorder (simulating the artifact of diffuse superficial FDG uptake seen on non-attenuation corrected images) [51]. Adenopathy associated with the disorder is generally FDG avid, but low grade uptake is of unceratin significance as benign dermatopathic lymphadenitis commonly occurs [51].
Anaplastic large cell cutaneous type TCL: The indolent disorder (85% 10-year survival) typically affects older adults and consists of a solitary lesion [46]. This is one of the least FDG avid TCL's [46].
Breast implant-associated anaplastic large cell lymphoma (BIAL): BIAL is characterized by two subtypes- the development of a late-onset peri-implant effusion (an effusion defined as occuring more than 1 year after implantation) or less commonly as a peri-implant mass [76]. The median time of onset is 10 years after exposure to a textured breast implant (commonly macrotextured) [76]. The lesion is felt to derive from chronic inflammation and persistent activation of T-cells reacting to bacterial biofilm arising in breast implants [76]. Patients present with a rapid and dramatic breast swelling as result of the effusion (85% of cases) or with a palpable mass adjacent to the prothesis in the affected breast (15% of cases) [76]. Approximately one-third of patients also report pain, and one-fourth of patients present with skin lesions- most commonly erythema [76]. The majority of patients have unilateral disease (with only two reported cases of bilateral disease) [76]. The presence of associated involved adenopathy at diagnosis is estimated to be 2-14% [76].
Unlike with many other lymphomas, surgical excision is recommended for primary treatment and is curative in most cases (with enblock excision and explantation of the breast implant with total capsulectomy and any associated masses, as well as contralateral prophylactic breast implant removal) [76]. The majority of patients with the peri-implant effusion subtype have indolent disease and a good clinical outcome [76]. The mass-forming subtype is associated with a poorer prognosis [76]. The overall survival has been reported to be 97% at 3-years and 92% at 5 years [76]. Patients with effusion only disease have achieve complete remission in 93% of cases (compared to 72% for the mass subtype) and decreased relapse rates (14% compared to 50% for the mass subtype) [76].
PET/CT has been reported to have a low sensitivity for the peri-implant effusion subtype (approximately 38%) and 64% for the mass subtype [76].
Natural killer nasal and nasal type TCL: Extranodal NK cell T-cell lymphoma, nasal type, is the most common peripheral T-cell lymphoma in Asian populations (40-74% of sinonasal lymphomas are NK/T-cell lymphomas in Asia [55]) and has also been referred to as angiocentric lymphoma and lethal midline granuloma [51]. This is an aggressive lesion more common in Asian men and there is a strong association with Epstein-Barr virus infection [46]. The lesion is confined to the nasal cavity or paranasal sinuses and is variably FDG avid [46,51]. The lesion typically has a good response to radiation therapy, but a poor response to conventional chemotherapy [55]. Identical tumors can develop in extranasal locations (and are designated "non-nasal type"- these can occur in the skin, GI tract, testis, kidneys, and respiratory system) [51]. Up to 83% of the lesions can be FDG positive [51]. T/NK-cell lymphomas have a worse prognosis compared to B-cell NHL. PET/CT has been shown to be superior to conventional staging methods for NK/T-cell lymphoma and can alter treatment planning in up to 44% of patients [55].
Enteropathy-type TCL: Enteropathy TCL is a rare lesion [51]. Affected patients typically have a history of gluten-sensitive enteropathy (celiac disease) or dermatitis herpetiformis and the disorder carries a poor prognosis [46]. FDG PET is more sensitive than CT in the detection of enteropathy-type TCL [46].
For a summary of FDG uptake in various histologic subtypes of NHL and HD click here.
Hodgkin's Disease
Hodgkin's disease is one of the most common malignant neoplasms in young Americans with 7500-8000 new cases annually [12,28]. The disease has a bimodal age distribution with a peak in the late 20's and then a second peak after the age of 50 years [28]. There are four subtypes of Hodgkin's disease: Nodular sclerosing (most common form), mixed cellularity (second most common form), lymphocyte predominant (favorable prognosis), and lymphocyte depleted (worst prognosis) [35]. With regards to FDG avidity, the nodular sclerosing subtype has the highest SUV, while the lymphocyte predominant subtype has the lowest SUV [49].
Hodgkin Reed-Sternberg cels escape immune surveillance through a genetic alteration in chromosome 9p24.1, leading to an overexpression of the programmed death 1 (PD1) ligands [71]. Pembrolizumab and nivolumab are PD1 blockade antibodies (anti-PD1) that restore immunity against Hodgkin Reed-Sternberg cells [71]. Anti-PD1 has recently demonstrated high response rates (64-87%) in relapsed or refractory Hodgkin lymphoma [71]. However, anti-PD1 therapy induces new patterns of disease response, with early findings that suggest progressive disease, but subsequent later response (pseudo-progression or indeterminate response on initial post-therapy imaging) [71]. In HD patients on anti-PD1 therapy, FDG tracer uptake in the healthy spleen is significantly increased in responders [71].
For Hodgkin’s disease, the stage at presentation and tumor cell type determine the patients overall prognosis and optimal method for treatment [28]. Early-stage HD has been routinely treated with 2-4 cycles of doxorubicin, bleomycin, vinblastine, and dacardazine (ABVD) chemotherapy and 20-30 Gy of involved field radiotherapy (combined-modality therapy has been shown to provide an improvement in disease control compared to chemotherapy alone) [70]. The treatment options for advanced stage HD include 6-8 cycles of ABVD or the more intensive escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPPesc), with consolidation radiotherapy to initially bulky sites or residual masses larger than 2.5 cm [70].
Because the anatomic extent of disease is the single most important factor influencing relapse-free duration and overall survival in patients with Hodgkin’s disease, accurate staging prior to initiation of therapy is essential for proper patient management [2]. The 5 year survival for stages I and IIA are close to 90%, and even in advanced stage disease (stage IVA or IVB) the survival can be as high as 70% [28].
Unfortunately, demonstration of all sites of disease can be difficult utilizing conventional staging methods. Between 20%-30% of patients with presumed localized supra-diaphragmatic disease at the time of initial presentation can be shown to have infra-diaphragmatic disease detected at staging laparotomy [5]. Unfortunately, laparotomy is invasive and associated with immediate and potential long-term complications --especially in post-splenectomy patients. The optimal staging method for lymphoma should be able to non-invasively identify all sites of disease.
Conventional imaging with CT or MR has been the primary means to evaluate and stage patients with lymphoma. These modalities can reveal anatomic abnormalities suggestive of tumor involvement. Conventional imaging is primarily dependent on lymph node size the determination of tumor involvement. Generally, lymph nodes greater than 1 cm in size are considered suggestive of tumor involvement (depending on anatomic location). Unfortunately, normal-sized lymph nodes can harbor malignancy and enlarged nodes may be reactive. Furthermore, infiltrative involvement of the liver, spleen, and bone marrow cannot be accurately detected by conventional imaging modalities.
Gallium-67 imaging has been utilized for the evaluation of lymphoma, but the agent suffers from several limitations. First, the gallium exam requires several days to complete (PET exams are performed about 1 hour following injection of the agent). Second, the tumor-to-background ratio is higher for PET than for gallium [29]. Although probably adequate for imaging of the chest, gallium suffers from decreased sensitivity for evaluation of the abdomen due to high physiologic liver and bowl activity [1]. Third, due to its lower anatomic and spatial resolution, gallium imaging has decreased capability to detect small lesions when compared to FDG-PET imaging [1,4]. As a result of these limitations, up to 36% of lesions seen on PET images may not be visible on gallium exams [29]. Also, negative post-therapy gallium scans are not useful unless a pre-therapy scan demonstrating tumor accumulation of the agent were performed [7]. Post-therapy scans can also suffer from non-specific hilar uptake of gallium that can be confused for recurrent disease [8]. Overall, FDG PET exams are more sensitive than gallium imaging in the evaluation of lymphoma patients [22,26].
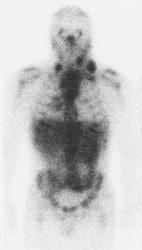
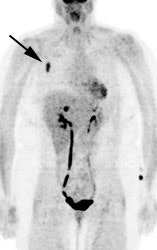
|
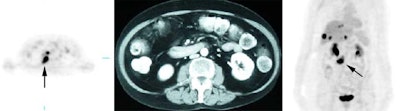
Gallium scan versus FDG PET exam: The exam on the left is from a gallium scan in a patient with lymphoma. Note the marked amount of background activity and lower spatial resolution compared to a FDG PET exam on an different lymphoma patient (back arrow points to nodal tracer uptake) |
|
|
FDG PET imaging for lymphoma:
PET is considered a reference standard for the pretreatment staging of FDG avid lymphomas (except chronic lymphocytic leukemia and small lymphocytic lymphoma, lymphoplasmacytic lymphoma and Waldenstrom macroglobulinemia, mycosis fugoides, and marginal zone NHL unless there is a suspicion of aggressive transformation [67].
There are three FDG lymphoma categories [83]-
1- Routinely FDG-avid lymphomas- Hodgkin, diffuse large B-cell, follicular, mantle cell, nodal peripheral T-cell, lymphoblastic, and Burkitt
2- Generally not FDG avid lymphomas (best evaluated by diagnostic anatomic imaging)- Small lymphocytic and chronic lymphocytic
3- Variable FDG uptake- some marginal zone lymphomas and some T-cell lymphomas such as cutaneous T-cell lesions
Compared to conventional imaging, FDG-PET imaging has certain advantages:
1- PET imaging provides a complete body survey which is important when evaluating a multi-focal disease process such as lymphoma [2].
2- FDG-PET imaging provides high lesion contrast which permits easy detection.
3- The exam provides very good anatomic localization due to the tomographic nature of the images [2]. PET/CT imaging further improves the accuracy of staging and response assessment over conventional anatomic imaging [37].
4- Lesion detection is based on a biochemical signal (increased metabolism) rather than anatomic criteria [2]. PET improves detection of occult splenic disease, bone lesions, and small tumor foci compared to CT [37].
The main trends in healthcare at the present time are increasing cost-effectiveness and decreasing the number of invasive procedures. Non-invasive, metabolic FDG-PET imaging can provide additional information regarding accurate staging, thereby reducing the number of surgical procedures required for staging [8]. PET imaging can also monitor patient response to treatment [8].
PET has been shown to have a major impact on management decisions for lymphoma patients [15]. PET findings can result in a change in patient staging in 8% to 44% of patients (either up-stage or down-stage) [1,4,5,10,15,34,35,64,73]. FDG-PET exams findings result in a change in patient management in 15 to 62% of patients [15,30,64,73]. In cases of discordant CT and PET findings- PET has been shown to be correct in up to 83% sites [34]. Importantly, it has also been demonstrated that for patients with aggressive non-Hodgkin lymphoma, initial PET/CT staging is associated with better survival/decreased mortality compared to staging by CT alone (despite frequently upstaging patients which suggests more appropriate adjustment of therapy based on the PET scan findings) [73,74]. PET imaging can be positive in up to 50% of patients felt to be in complete remission [15] and the implementation of FDG PET imaging can also result in long term cost savings [4]. Whole-body PET imaging followed by conventional imaging of areas of abnormal FDG uptake has been shown to be more cost effective than a standard conventional staging algorithm [4]. Total management costs were almost half compared with the conventional staging algorithm ($66,292 for conventional staging and $36,250 for FDG-PET staging) [2, 4]. With the advent of PET/CT- coregistered metabolic and anatomic data can be viewed simultaneously [30]. The use of PET/CT has been shown to be more sensitive and specific than CT imaging alone for staging and restaging lymphoma (particularly for organ involvement) [30].
For patients with DLBCL baseline metabolic tumor burden carries prognostic information [82].
Nodal disease:
PET imaging with FDG has demonstrated high sensitivity for the detection of abnormal lymph nodes in patients with lymphoma [2]. PET imaging will generally detect all abnormal lymph nodes identified on CT [1]. Additional sites of nodal disease are commonly detected, particularly in the abdomen where CT may miss small mesenteric lymph nodes [1].
Reported sensitivities for PET FDG in detecting sites of nodal disease range from 62%-100%, but the studies are very heterogeneous and tend to compare patients with various histologic grades of lymphoma [4, 8]. Despite this variability, FDG has consistently been shown to be more accurate than CT in staging lymphoma [3]. This is because PET imaging can detect additional lesions (nodal and extra-nodal) which are not identified by conventional imaging [4, 5].
Extranodal disease:
Extra-nodal organ involvement (spleen, liver, and bone marrow) carries a worse prognosis when compared to patients with isolated nodal disease [5]. FDG-PET imaging can detect up to 57% more extra-nodal sites of disease than noted on CT scanning [5].
Spleen and Liver Involvement with Lymphoma:
In Hodgkin's disease hepatic and splenic involvement occur in 3.2% and 23% of patients, respectively [5]. For NHL, hepatic and splenic involvement are seen in 15% and 22% of patients, respectively [5]. However, other authors quote a much higher prevalence of splenic involvement at presentation (30-40% of Hodgkin's and 10-40% for non-Hodgkin's) [44]. In 10% of patients with Hodgkin's disease the spleen is the only infradiaphragmatic site of disease [44]. In the Lugano classification system, splenomegaly is defined as size greater than 13 cm in vertical length and this is suggestive of splenic involvement [64]. However, infiltrative involvement of the liver, spleen, and bone marrow cannot be accurately detected on conventional imaging, as organ size is a poor predictor of tumor involvement [5]. In fact, approximately 30% of patients with splenic enlargement do not have malignant involvment [5]. Sensitivity rates for CT range from 15%-37% for infiltrative splenic disease, and from 19%-33% for infiltrative liver disease [5]. Reported sensitivities for PET are between 80-100%, and the specificity has been reported to be 100% [44].
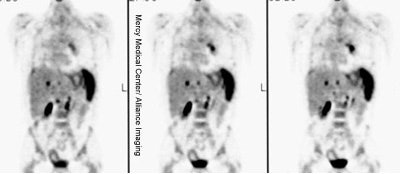
|
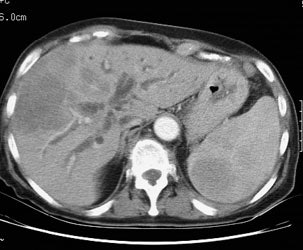
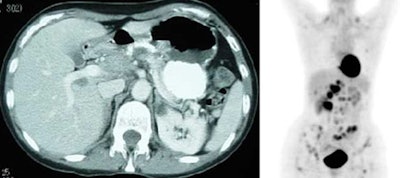
Diffuse splenic involvement: The patient shown below has non-Hodgkins lymphoma. The FDG PET exam demonstrated multiple sites of disease including diffuse involvement of the spleen, focal liver involvement, and persistent sites of adenopathy in the chest and abdomen. Case courtesy of Mercy Medical Center Alliance Imaging, Dr. Steve Allen. |
|
|
FDG PET imaging is more accurate than CT for the detection of lymphomatous involvement of the spleen [5,27]. On FDG PET imaging, diffuse or focal splenic uptake of greater intensity than the liver is felt to represent tumor involvement [27]. Infiltrative lymphomatous involvement of the spleen can be detected with a 67% increased frequency when compared with CT imaging [5]. Hepatic tumor involvement is characterized by focal or diffuse increased tracer accumulation [5].
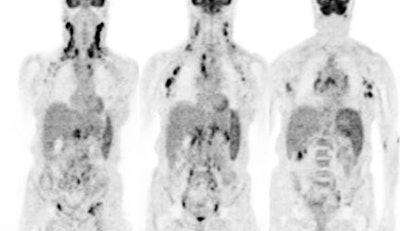
|
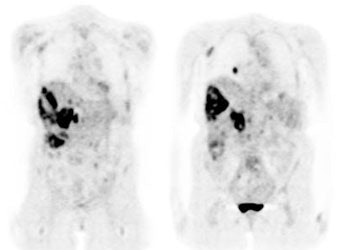
Diffuse lymphoma: In the case below, FDG PET imaging was performed prior to conventional imaging for patient staging. There is widespread tracer uptake within adenopathy in the neck, chest, abdomen, and pelvis. The spleen is enlarged and the intensity of FDG uptake is greater than the liver (SUV was 2.5) which is indicative of infiltrative splenic involvement. There was no evidence of osseous disease. (Click image to view rotating cine file) |
|
|
|
Liver involvement: The FDG PET study below demonstrates hepatic involvement in a patient with widespread lymhoma. The CT scan in this case was positive. Splenic involvement is also evident on the CT scan and was also present on separate slices (not shown) from the FDG study. (Click PET image to view rotating cine file) |
|
|
Bone marrow involvement with lymphoma:
Bone marrow involvement indicates stage IV disease and carries a less favorable prognosis for patients with NHL [11]. Bone marrow involvement in newly diagnosed lymphoma occurs in 5%-15% (average 10%) of patients with Hodgkin’s disease and in 19%- 83% (average 25%) of patients with NHL (involvement is seen in 50-80% of patients with low grade NHL and 25-40% of those with high grade NHL) [5 6,10,11,35,49].
To be detected on CT imaging, osseous involvement needs to be focal and associated with bone destruction. Patients with infiltrative marrow involvement demonstrate no bone destruction and are usually asymptomatic [5]. Both focal and infiltrative osseous lesions can be detected on FDG-PET imaging [5].
|
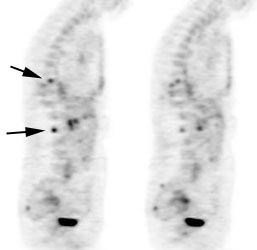
Osseous lymphoma: Focal osseous involvement of the spine was identified in this patient with lymphoma. Conventional images were negative. |
|
|
In comparison to 99mTc-MDP bone scintigraphy, FDG-PET imaging has been shown to be superior for the evaluation of osseous involvement by lymphoma [9]. FDG imaging can detect up to 42% more osseous lesions than 99mTc -MDP bone scintigraphy [9]. Lymphomatous involvement of the bone marrow can be detected on FDG-PET with a sensitivity of up to 81%- 88%, and a specificity of up to 100% [1, 6, 10]. Infiltrative osseous involvement appears as generalized increased tracer activity greater than the liver within the bone marrow [5,24]. However, other authors suggest that strong diffuse marrow uptake often represents an unspecified metabolic marrow activation or simply a hyperplasia of the hemopoietic cell compartment [69]. Such non-pathologic diffuse uptake is associated with a lower hemoglobin level, younger age, bulky disease, and lower albumin levels [69].
Bone marrow biopsy (bilateral posterior iliac crests) represents the standard diagnostic procedure to confirm bone marrow involvement [6]. Unfortunately, bone marrow biopsy samples only a small volume and is associated with a high rate of false-negative findings (due to patchy or localized marrow involvement) that may lead to errors in patient management [6]. In a meta-analysis comparing PET and bone marrow biopsy (BMB), FDG PET imaging had a sensitivity of 51% (95% CI 38-64%) and a specificity of 91% (95% CI 85-95%) [33]. There was better sensitivity for patients with Hodgkin disease and aggressive histiologic non-Hodgkin's lymphoma [33]. In another article comparing PET CT versus BMB, PET was shown to be more sensitive (94% VS 24%), had a higher negative predictive value (98% vs 80%), and was more accurate (98% ve 81%) [57]. PET/MRI may have better ability to detect bone marrow involvement [62]. However, up to 5.6% of patients with negative PET imaging for marrow involvement can have positive bone marrow biopsy results (false negative PET imaging) [69].
FDG PET has good, but not excellent, concordance with bone marrow biopsy and PET imaging can aid in the detection of unsuspected sites of osseous involvement which may not be identified on routine bone marrow biopsy [33].
|
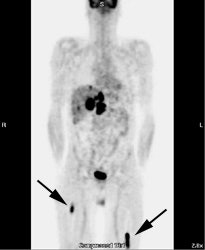
Osseous lymphoma: Unsuspected focal osseous involvement of the femurs was identified on PET imaging of this patient with newly diagnosed B-cell non-Hodgkin's lymphoma (black arrows). Extensive hepatic involvement was seen within the abdomen, but there were no other sites of bone disease. Focal bone disease such as this will not be detected on bone marrow biopsy. |
|
|
In one study, PET imaging revealed osseous involvement not detected on bone marrow biopsy in 12.8% of patients [6]. This finding resulted in an increase in patient stage in 10% of patients [6]. In that same study, bone marrow biopsy revealed tumor involvement in 5% of patients that was not detected by FDG-PET imaging [6]. However, the cases in which FDG-PET was false negative involved low- or intermediate-grade lymphomas with only discrete displacement (up to 10%) of normal marrow [6]. Because the degree of FDG uptake declines in a lower grade of malignancy -- the combination of lower numbers of malignant cells and low FDG tumor uptake likely resulted in low marrow activity [6]. Therefore, PET imaging complements but does not replace bone marrow biopsy [37].
Other applications for PET imaging in the evaluation of lymphoma include: Assessment for tumor recurrence, differentiate scar from residual tumor mass, and to monitor response to therapy and predict outcome.
1- Assess for tumor recurrence: PET provides a rapid whole body survey to assess for recurrent disease.
|
Recurrent lymphoma: The patient shown below had a history of submandibular lymphoma and presented for the evaluation of right hip and back pain. CT imaging revealed evidence of recurrent lymphoma, but failed to identify the lesion in the right psoas muscle which was likely the cause of the patient's pain (black arrow on FDG PET exam). Case courtesy of CTI PET Systems, Inc. |
|
|
2- Monitor response to therapy and predicting outcome:
Changes in glucose metabolism often precede structural changes in tumor size which can enable earlier detection of response [85]. Earlier response assessment (responder vs non-responder) can aid in adjusting therapy [85].
The International Workshop Criteria for assessment of tumor response in NHL have been revised to include 18F-FDG [39]. A complete response is now based primarily on the PET scan findings [39]. Assessment for response to treatment can be performed qualitatively on visual analysis (Juweid criteria) or quantitatively with SUV measurements. For qualitative assessment, lesions are considered PET positive only when their intensity is higher than that of mediastinal blood pool activity (if the lesion is larger than 2 cm) [39,43,50]. Other authors consider minimal residual uptake (defined as activity less than, equal to, or only slightly higher than mediastinal blood pool activity) as negative [52]. On interim PET imaging performed after two cycles of chemotherapy, this minimal residual uptake is felt to be caused by an inflammatory response in the tumor [56]. An exception is made for lesions with a diameter of less than 2 cm, because partial volume effects can result in underestimation of activity [39]. Hence, any uptake above surrounding background is considered positive for lesions under 2 cm [39,43,50,56]. Use of a reference such as blood pool activity is useful, especially if patients do not have a baseline exam from which to measure changes in SUVmax [53]. However, false positive interim PET scans can occur in 30-70% of cases- likely related to an inflammatory response [56] and some authors suggest that using absolute SUVmax reduction is more accurate than visual analysis [58]. Also, treatment with the monoclonal antibody rituximab as part of the R_CHOP regimen in diffuse B-cell lymphoma may induce a strong inflammatory "flare response" and can result in false-positive findings on interim PET imaging [64].
When monitoring patients for response to therapy it is important to remember that there are statistical variations in tumor SUVmax measurements. Repeat measurements of the same lesion can vary by 8%-14% [14]. Changes of greater than 25% in SUV max cannot be explained by statistical variations alone and should be considered to represent a definite change in metabolism [14]. The European Organization for Research and Treatment of Cancer (EORTC) defines progressive metabolic disease as an increase in tumor SUVmax greater than 25%, a visible increase in the extent of FDG tumor uptake of greater than 20% in the longest dimension, or the appearance of new foci of tracer uptake [48]. Stable metabolic disease is defined as an increase in SUV max of less than 25%, or a decrease of less than 15% [48]. A partial tumor response is defined as a decrease in SUVmax of 25% or more, and a complete response is indicated by resolution of FDG uptake that becomes indistinguishable from surrounding normal tissues [48].
The optimal time to obtain a post-therapy scan has not been completely resolved [42]. In general, it is recommended that PET imaging be performed not earlier than 3 weeks (preferably 6-8 weeks) after completion of chemotherapy and not earlier than 8-12 weeks following radiation therapy [39,43,49,50,56]. However, a minimum wait of 10 days after chemotherapy has also been recommended [42].
For interim PET imaging, the scan should be scheduled at least 2 weeks, and preferably 3 weeks, after the initiation of therapy, or 4-5 days before the start of the subsequent therapy cycle [56].
Following completion of first-line chemotherapy:
Obtaining a complete remission is the main objective of first-line chemotherapy because it is usually associated with a longer progression-free survival compared to a partial remission [25]. Conventional imaging modalities such as CT and MR are capable of demonstrating only decrease in lesion size and are poor predictors of clinical outcome following treatment for lymphoma [18]. On long-term follow-up, 50% or fewer of patients with positive CT findings have disease relapse or other evidence of residual tumor [18].
Currently, FDG PET is considered to be more accurate than anatomic imaging modalities in assessing treatment effects [19,25]. The optimal time to obtain a post-therapy scan has not been completely resolved, but a minimum of 10 days after chemotherapy has been recommended [42].
On PET imaging, FDG activity reflects the mass of viable cells within a tumor [4]. Following initiation of chemo or radiation therapy, changes in tumor metabolism occur prior to any significant decrease in tumor mass (size) [4]. A negative FDG PET scan after the end of treatment excludes residual viable tumor with high certainty in both Hodgkin lymphoma and diffuse large B-cell lymphoma (with a higher negative predictive value in Hodgkin lymphoma) [64]. Persistent FDG uptake after completion of chemotherapy is an indication of lack of tumor response and is associated with a shorter progression-free survival [19,20,21,23,28,32].
Response assessment is a continuous spectrum, but for convenience is divided into distinct groups- complete response, partial response, stable disease, and progressive disease [85].
The National Comprehensive Cancer Network treatment guidelines use the Deauville criteria to guide therapy during mid-treatment and at end-of-treatment assessment [66].
Deauville score:
1- No uptake above background
2- Uptake less than or equal to the mediastinal blood pool
3- Residual lesion uptake that is greater than mediastinal blood pool, but less than or equal to the liver
4- Uptake moderately more than the liver at any site
5- Markedly increased lesion uptake (2-3x's higher than SUVmax in the normal liver) or new foci of uptake suspicious for lymphoma X- New areas of uptake unlikely to be related to lymphoma
The Lugano Classification expands on the Deauville scores for response assessment-
The PET results are considered negative (complete metabolic response) when the residual lesion has FDG uptake less than or equal to that of the mediastinal blood pool (Deauville 1 or 2)- a finding which may permit de-escalation of therapy [66,78].
PET results are positive (no metabolic response) when there is residual uptake higher than the liver (Deauville 4 or 5) - findings which may permit escalation of therapy [66,78].
Partial response seen on interim PET indicates disease response, but denotes residual disease is present at end of treatment [85].
Residual lesion uptake that is greater than mediastinal blood pool, but less than or equal to the liver (Deauville 3) on interim assessment likely represents a complete metabolic response in patients receiving standard treatment [67,85]. However, in clinical trials a score of 3 may be regarded as inadequate response to avoid under-treatment [67] (but also negative for considering escalating therapy [66]).
| Complete
response |
Partial response |
No response/stable disease |
Progressive disease |
| Score of 1 or 2 (likely 3, but see above) |
Score of 4 or 5 with decreased uptake
compared to baseline |
Score of 4 or 5 without significant change
from baseline |
Score of 4 or 5 increased in comparison to
baseline or new sites of FDG avid disease |
| No FDG avid bone marrow disease |
Residual bone marrow uptake above background
at involved site, but reduced from baseline |
No change in marrow uptake from baseline |
New or worsening areas of bone marrow uptake |
| No new FDG avid lesions |
No new FDG avid lesions |
No new FDG avid lesions |
In a meta-analysis, for HD the pooled sensitivity and specificity of FDG PET imaging for detection of residual disease after completion of first line therapy was were 84% (CI 71-92%) and 90% (CI 84-94%), respectively [41]. In patients with positive FDG PET scans following completion of first-line chemotherapy, relapse can be observed in 62% to 100% of patients [19,20,25,28,32]. A negative PET scan following therapy is associated with a much lower risk of relapse (4% to 16%) and a longer disease free survival [19,20,25,28]. However, the risk of recurrence maybe somewhat higher with residual disease on CT that measures greater than 2 cm (even when the FDG PET scan is negative for metabolic activity) [64].
In a study of patients with Hodgkin's disease following completion of therapy [28], the overall diagnostic accuracy of FDG PET imaging for the prediction of disease relapse was 92%, compared to 56% for conventional CT imaging [28]. PET had an overall sensitivity of 79%, a specificity of 97%, a positive predictive value of 92%, and a negative predictive value of 92% [28]. In this study, 92% of patients with a positive PET scan following therapy, had a relapse detected on follow up imaging [28]. Three patients with negative post-therapy scans did eventually relapse, but the time to relapse was approximately 1 year [28].
In another study, the relapse rate was 17% versus 25%, for PET versus CT negative patients, and 100% versus 41%, for PET versus CT positive patients [32]. Mid-treatment imaging is also commonly performed and the negative predictive value of a mid-treatment scan (i.e.: the probability of patients with a negative PET result achieving durable remission) has been consistently high (at least 94%) [37]. However, the positive predictive value (i.e.: for disease progression) has been more variable (between 62-90%) [37].
In another study of patients with Hodgkin's disease [38], a negative PET scan was associated with a good prognosis and disease free interval [38]. However, PET scans were false positive for residual disease in 15% of patients [38]. In 75% of the cases of false-positive exams, the patients had received combination radiation and chemotherapy and the false positive findings were within the radiation field [38]. This study emphasized the need for biopsy of sites of FDG accumulation to confirm residual disease- particularly in the subset of patients receiving chemo-radiation [38].
A Deauville score of 4 or 5 on interim PET carries prognostic significance with a reported median progression-free survival of only 17 months, compared to greater than 6 years for patients with scores or 1 to 3 [78].
In a study of pediatric HD patients, the use of the Deauville score for post therapy scan interpretation was shown to improve the clinical utility of end-of-chemotherapy PET by an increase in PPV from 44% to 73%, and the score is associated with a very high NPV over 95%) [75].
FDG PET imaging can also be used to monitor the effectiveness of radioimmunotherapy [42]. Patients with a greater than 52% drop in SUV max have a longer survival compared to patients with lesser declines (larger declines are associated with longer disease free survival) [42]. In patients who progress following radioimmunotherapy, it appears that new sites of disease commonly develop, as opposed to recurrence or progression at previous sites of disease [42].
However, for interim PET imaging after two cycles of chemotherapy, other authors have suggested that quantitative metrics (change in SUVmax) are superior to the Deauville score for outcome prediction [80]. In DLBCL thresholds of 66% SUVmax reduction after two cycles of rituximab, cyclophoasphamide, doxorubicin, vincrinstine, and prednisone (R-CHOP) and 73% after 4 cycles of R-CHOP lead to maximal separation of survival curves between good and poor responders [80]. After two cycles of R-CHOP, SUVmax reduction had a PPV of 56.6% vs 38.4% for the Deauville score [80].
In a study of patients with DLBCL, an interim PET scan demonstrating Deauville 4-5 disease or a change in SUVmax of less than or equal to 70% predicted an extremely poor prognosis [84]. Baseline MTV predicted both PFS and overall survival [84].
Of clinical significance is that most patients that achieve durable remissions will have negative PET scans after the first few cycles of chemotherapy [37].
Early prediction of response to therapy:
Studies are exploring the early implementation of FDG PET imaging to assess for treatment response. Early assessment of response to chemotherapy can help to avoid the toxicity and cost of ineffective treatment [4]. Second-line therapy can then be instituted early to maximize the patient’s potential for cure [4, 17]. A significant decrease in FDG uptake can be seen as early as one day following initiation of chemotherapy (therefore baseline scanning must be performed prior to institution of therapy) [31]. FDG PET imaging has been evaluated following the first cycle of chemotherapy, after two cycles, and after 4 cycles of treatment.
FDG-PET imaging performed after completion of the first course of chemotherapy can provide important information regarding treatment outcome [17]. Early data indicates that following effective chemotherapy, there is a rapid decrease in metabolic activity within the tumor (a decrease in SUV between 75%-90% within 7 days of initiation of therapy) [4]. Patients that demonstrate persistent positive FDG exams after one cycle of chemotherapy are at a greatly increased risk for subsequent disease relapse [17]. Recurrent disease occurred in 90% of patients with positive FDG exams after their first course of chemotherapy [17]. Conversely, 85% of patients with negative FDG exams after one course of therapy remained in remission after a minimum follow-up of 18 months [17]. However, at least one study has suggested that up to 54% of patients with residual activity on interim PET scans can achieve a complete remission at the end of chemotherapy [59]. These patients have an overall good prognosis, although slightly lower than patients with negative interim PET scans (2 year EFS of 86% and OS of 92%, versus 97% and 97%, respectively) [59]. FDG uptake in the tumor mass in these patients may be due to an inflammatory reaction rather than residual viable tumor [59].
Persistent positive FDG exams after one cycle of chemotherapy likely reflect metabolic activity within potentially resistant clones, which do not respond to treatment as quickly as chemo-sensitive cells [17]. Imaging after one course of chemotherapy was also more accurate than imaging following completion of therapy for predicting outcome (87% versus 70%) [17].
Most interim assessment exams are performed following two cycles of treatment [68,79]. After 2 cycles of chemotherapy, patients with favorable outcomes have been shown to have a greater decrease in tumor SUV [45] (an early complete metabolic response is associated with an event-free survival in excess of 80% [77]). In one study, after 2 cycles of ABVD, treatment failure occurred in 53% of PET positive patients and in only 8% of PET negative patients [52]. Various changes in SUVmax have been reported to be associated with improved prognosis. A reduction in SUV max in the hottest tumor lesion by more than 66% from baseline after two cycles of chemotherapy in diffuse B-cell lymphoma (or 77% after 4 cycles) indicates satisfactory response to therapy and a good prognosis [64,79]. A decreased in SUV max of greater than 66% from baseline has been associated with an event-free survival of 79%, compared to a 21% event free survival in patients with less than 66% SUV max reduction [45]. Another study found a decrease in SUVmax of 48.9% to be highly predictive of outcome with 100% specificity [77]. ΔSUVmax of greater than 66% also appears to be a more accurate reflection of tumor response compared to Deauville scoring system which is associated with a higher false positive rate (and more inter-observer variability) [79]. In one study, the number of patients with an unfavorable interim PET response was 4 times higher using the Deauville score compared to the ΔSUVmax (which had a higher specificity of 88%, compared to 57.5% for the Deauville score [79]. With a high false positive rate, the Deauville score may result in unnecessary treatment intensification in patients that would have responded satisfactorily to standard therapy (and that treatment could be associated with higher costs and greater toxicities) [79].
For Hodgkin lymphoma a change in SUV max of 71% after two cycles of chemotherapy has been proposed to indicate a satisactory response [64].
After 4 cycles of treatment, using visual analysis, patients with negative PET studies have an event-free survival of 82%, compared to 25% in PET positive patients [45]. When using visual assessment, imaging after 4 cycles of treatment may provide better prognostic information as 42% of patients that were considered positive after 2 cycles of treatment became PET negative by 4 cycles (with an event rate of about 8%) [45].
|
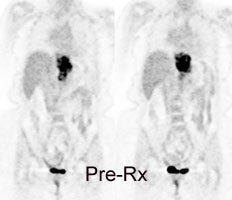
Monitor response to therapy in lymphoma: The patient shown below was a 29 year old male with a history of recurrent lymphoma despite chemotherapy and stem cell transplant. The patients initial PET scan (left) demonstrated extensive nodal disease throughtout the neck, chest, abdomen, and pelvis. The patient was treated with a repeat stem cell transplant. A post-treatment FDG PET exam demonstrated interval resolution of previously identified sites of disease consistent with a response to therapy. Note renal collecting system activity on both studies. The exams were performed on a Siemens ECAT EXACT PET scanner (manufactured by CTI). Case courtesy of North Texas Clinical PET Institute, Dallas, Texas and CTI PET Systems, Inc. Click images to view cine avi file (230K) |
|
|
|
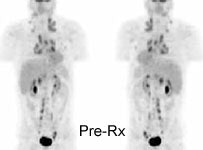
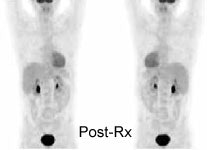
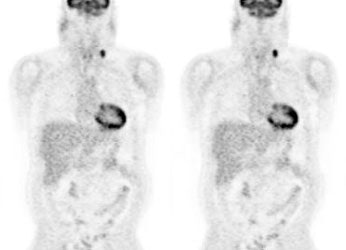
Monitor response to therapy in lymphoma: The patient shown below is a teenage male with Hodgkins disease. The pre-therapy scan confirmed disease only above the diaphragm. The post-therapy scan was performed following two cycles of chemotherapy and demonstrated complete resolution of all sites of disease. Early data indicates that following effective chemotherapy, there is a rapid decrease in metabolic activity within the tumor. Click images to view rotating avi files (note para-cardiac and costophrenic angle nodes can also be seen on these images). |
|
|
|
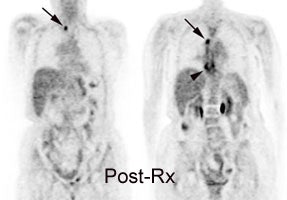
Monitor response to therapy in lymphoma: The patient shown below had a history of Hodgkins lymphoma and had undergone stem cell transplant. A follow-up CT scan revealed increased soft tissue in the region of the porta hepatis which was suspicious for adenopathy, but the patient's lack of internal fat limited the CT evaluation. The patients FDG PET exam revealed widespread intra-abdominal, pelvic, and inguinal adenopathy consistent with extensive recurrent lymphoma which was not appreciated on the CT exam. Case courtesy of CTI PET Systems, Inc. |
|
|
It is important to keep in mind that a negative PET result does not indicate a total eradication of disease, but it does imply a certain amount of cell killing (between 2 and 10 log units of tumor cell killing) [37]. Thus, patients with negative mid or post treatment scans represent a heterogeneous group in terms of relapse risk [37]. A negative post first cycle or mid-treatment scan may imply a greater likelihood for cure as these patients will receive additional chemotherapy which will result in additional cell killing [37].
Prior to allogeneic and autologous stem cell transplant:
Stem cell transplant therapy is costly and associated with morbidity and mortality [65]. Thus, there is a need to identify patients with having a high likelihood for a successful outcome [65]. In patients with aggressive lymphomas, FDG PET/CT imaging prior to stem cell transplant has been shown to habe prognostic value for predicting recurrence following therapy [65]. The presence of FDG avid lesions prior to stem cell transplant indicates a lower likelihood for success of the treatment (the association between FDG PET findings and outcomes is stronger in patients who undergo autologous rather than allogeneic transplants) [65]. The risk of post transplant recurrence correlates with the highest lesional SUVmax [65].
3- Differentiate scar from residual tumor mass: In patients with lymphoma, between 30% to 64% will exhibit a residual mass following completion of therapy [4,23,25]. A residual mass after treatment of lymphoma is a clinical challenge because it may represent residual viable tumor or tissue fibrosis [7]. However, only 18% of patients with residual masses will eventually relapse [7,19]. Unfortunately, there are no reliable radiographic characteristics that permit differentiation between malignant and fibrotic tissue. A delay in the diagnosis of residual disease can result in a loss of precious time prior to institution of salvage therapy [23]. FDG will accumulate in viable tumor, but does not accumulate in fibrotic or necrotic tissue [4]. Compared to CT, FDG imaging has been shown to have a higher specificity (92% vs 17%), accuracy (96% vs 63%, and positive predictive value (94% vs 60%) in the evaluation of residual mass [35].
FDG accumulation within a residual mass is associated with a higher predictive value for relapse and an overall worse survival compared to patients without FDG uptake [7,23]. The disease-free survival at one year for PET-negative and PET-positive patients at one year has been reported to be 95% and 40%, respectively [23]. Persistent metabolic activity within a residual mass should prompt strong consideration for additional therapy [7], although in some cases the uptake may be related to thymic hyperplasia or a histiocytic reaction [23]. On long term follow-up, between 16% to 25% of patients with negative PET scans and a residual mass do eventually relapse [7,23], however, the relapse occurs in another site in up to 80% of these cases [7].
|
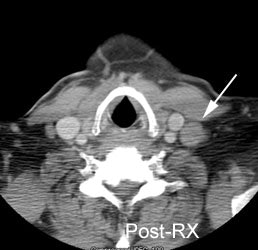
Residual mass post-therapy: The patient shown below had received chemotherapy for lymphoma. There was a residual left neck mass following completion of therapy. A FDG PET exam revealed persistent metabolic activity within the mass concerning for residual tumor. Based upon the FDG exam findings, the patient's management was changed to include additional chemotherapy. |
|
|
Limitations of FDG PET Imaging in Lymphoma:
FFalse-positives exams have been reported in association with non-specific inflammatory lymph nodes and this can potentially result in incorrect patient staging [1, 2]. However, it should be pointed out that conventional imaging could also produce false-positive exams when a reactive enlarged node is classified as involved with tumor. Lack of increased metabolic activity within enlarged nodes has been shown to correlate with lack of tumor involvement [4].
Small lesions (under 1 cm in size) may lack sufficient metabolic activity to be properly identified [2]; however, using this size criterion, nodes less than 1 cm in size would also not be characterized as abnormal by conventional imaging.
PET imaging can be degraded by physiologic activity that can mask lesions -- although to a much lesser degree than gallium scintigraphy. The brain has intense uptake of FDG and may obscure a central nervous system (CNS) lesion. As standard staging with CT does not usually include the brain, the decreased sensitivity of FDG-PET for this region is not a true limitation of the exam. With either conventional or PET staging, concern for CNS involvement would require further evaluation with MR imaging.
Cardiac uptake is high in a post-prandial state, and therefore patients should be fasting to minimize myocardial uptake and maximize evaluation of the chest. Renal and bladder activity (due to urinary excretion) may obscure abnormalities in the abdomen. Faint bowel activity can also be seen on FDG exams, but is generally not enough to cause confusion with actual disease.
Steroids can lead to lower FDG accumulation in malignant lymphoma than in the surrounding tissue as a result of their anti-insulin effect on carbohydrate metabolism [13].
Chimeric antigen receptor T-cell therapy:
Chimeric antigen receptor (CAR) T-cell therapy is an emerging for of cancer immunotherapy [87]. It relies on the harvest and manipulation of a patient's autologous T-cells [87]. Cells are transferred to a laboratory and are genetically modified using a disarmed virus to express a recombinant CAR of the cell surface, which can recognize and bond to specific proteins or antigens on the surface of cancer cells [87]. The artificial CAR construct is designed to combine both antigen-binding and T-cell signaling functions, which allows the cell to activate its cytotoxic pathway [87]. The CAR T-cells are infused back into the patient, where they bind to cancer cells that express the target antigen [87]. This activates CAR T-cells to eliminate cancer cells and gives rise to a powerful anti-tumor response [87]. The most success in treatment has been obtained with CAR T-cell therapy directed at the CD 19 antigen- a surface protein expressed by B-lymphocytes and malignant B-cells [87]. Initial trials have reported response rates of 50-80% in patients with large B-cell lymphoma (LBCL) in whom two or more lines of standard immunochemotherapy had failed, with 30-40% of patients achieving long-term remission and potential cure [87].
Prognosis:
PET imaging can be used to evaluate therapy response and PET exam findings carry prognostic significance [87]. Most patients undergo bridging therapy to stabilize disease during the 2-5 weeks when CAR T-cells are being manufactured [87]. This bridging therapy typically consists of systemic immunotherapy and chemotherapy or radiation therapy [87]. A focrable response to bridging therapy assessed by PET imaging has been found to be predictive for long-term survival after CAR T-cell therapy in LBCL [87].
Patients with a high MTV before CAR T-cell therapy for LBCL have been shown to have a worse outcome [87]. In one study, patients with a total MTV greater than 80 mL before treatment were at higher risk for early disease progression [87]. A lesion SUV max of at least 10, a lesion cross sectional area of at least 2 cm2, or a metabolic volume of at least 20mL were found to predict risk for local failure after CAR T-cell therapy and these findings may help to identify tumor sites that warrant radiation therapy bridging therapy [87].
The majority of progressive events (80-90%) will occur in the first 6 months after treatment [87]. Pseudoprogression (flare) is rarely observed after CAR T-cell infusion and if measurable tumor growth is seen at the one month followup PET scan, this most often reflects true disease progression [87].
Patients with a partial response at one month (Deauville score of 5) have dismal outcomes and CAR T-cell therapy should be considered as having failed [87]. Patients with refractory disease after CAR T-cell therapy often deteriorate rapidly [87].
At three months after infusion, a favorable response (partial or complete response) is highly predictive of long-term remission [87]. Patients with a complete response at 3 months have a70% or greater chance of remaining disease free at 2 years [87]. Patients who achieve a complete response (Deauville score of 1-3) at 6 months after CAR T-cell therapy have a favorable prognosis and a negative scan is a prerequisite for long-term remission and cure [87]. Patients with only a partial response at 6 months have a shorter duration of response and shorter progression-free survival [87].
CAR T-cell therapy complications:
Cytokine release syndrome (CRS): CRS is the most common adverse event after CAR T-cell infusion [87]. CRS happens when antigen recognition by CAR T-cells triggers the release of proinflammatory cytokines such as interleukin-6 and interleukin-1, resulting in systemic immune activation [87]. Symptoms usually develop within days of cell infusion [87]. The severity of CRS can range from mild to life-threatening with fever, hemodynamic instability, respiratory failure, capillary leak, and consumptive coagbulopathy [87].
Treatment is supportive with vasopressor and ventilatory support [87]. Tocilizumab is a humanized monoclonal antibody that inhibits interleukin-6 from binding its receptor, thereby reducing the cytokine storm and it can be used to treat both CRS and concurrent CRS/ICANS [87]. Steroids are also used to treat more severe CrS [87].
Immune effector cell-associated neurotoxicity syndrome (ICANS): ICANS is the second most common adverse event and can be seen independently or in conjunction with CRS [87]. The syndrome develops ni the first two weeks after infusion, although cases can occur after several weeks [87]. Proinflammatory cytokines are implicated in CNS inflammation, disruption of the BBB, and capillary leak [87]. The majority of cases are mild and self-limiting with a median durationof 7-14 days [87]. Common manifestations of ICANS include encephalopathy, HA, tremor, and focal weakness [87]. Expressive dysphasia may be a more specific sign in the early stages and it can progress to global aphasia [87]. Imaging findings include patchy areas of increased signal in the cerebral white matter on T2 and fluid-attenuated inversion recovery images [87]. Additional findings include increased signal on T2 images in the dorsal brainstem, thlamuc, basal ganglia, limbic system, and external capsule [87].
Isolated ICANS is treated with steroids [87]. Anakinra is an anti-interleukine-1 receptor antagonist that may reduce the effect of circulating cytokines in the management of steroid-refractory neurotoxicity [87].
Other PET agents in lymphoma:
68Ga-FAPI:
68Ga-FAPI has been found to be inferior to FDG imaging in the evaluation of lymphoma (staging accuracy 86% compared to 98% for FDG) and there were 2939 68Ga-FAPI negative/FDG positive lesions [86]. Another study also demonstrated lower accuracy (89% vs 100% for FDG) [88].
18F-fludarabine:
18F-fludarabine is a lymphoma specific adenine nucleoside analog resistant to adenosine deaminase [72]. The agent has demonstrated a high affinity for CLL and indolent non-Hodgkin lymphoma [72]. Tracer uptake is cell cycle independent [72]. Uptake of the agent has also been shown to be inflammation-independent [72].
Biodistribution: There is no uptake of the tracer in the brain or heart [72].
Limitations: The agent has been shown to miss lymphomatous infiltration in the testis- likely related to the role of the testis barrier [72].
REFERENCES: (1) Radiology 1997; Moog F, et al. Lymphoma: role of whole-body 2-deoxy-2-[F-18]fluoro-D-glucose (FDG) PET in nodal staging. 203: 795-800
(2) J Nucl Med 1997; Hoh CK, et al. Whole-body FDG-PET imaging for staging of Hodgkin's disease and lymphoma. 38: 343-348
(3) J Nucl Med 1999; Delbeke D. Oncological applications of FDG PET imaging: Brain tumors, colorectal cancer, lymphoma, and melanoma. 40: 591-603
(4) Clinical Positron Imaging 1998; Romer W, et al. Positron emission tomography in diagnosis and therapy monitoring of patients with lymphoma. 1 (2): 101-110
(5) Radiology 1998; Moog F, et al. Extranodal malignant lymphoma: Detection with FDG PET versus CT. 206: 475-481
(6) J Clin Oncology 1998; Moog F, et al. 18-F-Fluorodeoxyglucose-positron emission tomography as a new approach to detect lymphomatous bone marrow. 16 (2): 603-609
(7) Blood 1999; Jerusalem G, et al. Whole-body positron emission tomography using 18-F-Fluorodeoxyglucose for post-treatment evaluation in Hodgkin's disease and non-Hodgkin's lymphoma has higher diagnostic and prognostic value than classical conventional tomography scan imaging. 94 (2): 429-433
(8) Acta Oncol 1999; Bangerter M, et al. Positron emission tomography with 18-Fluorodeoxyglucose in the staging and follow-up of lymphoma in the chest. 38 (6): 799-804
(9) J Nucl Med 1999; Moog F, et al. FDG PET can replace bone scintigraphy in primary staging of malignant lymphoma. 40 (9): 1407-13
(10) Ann Oncol 1998; Bangerter M, et al. Whole-body 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) for accurate staging of Hodgkin's disease. 9 (10): 1117-22
(11) Clin Nucl Med 2000; Lee J, et al. Dichotomy between Tc-99m MDP bone scan and Fluorine-18 fluorodeoxyglucose coincidence detection positron emission tomography in patients with non-Hodgkin's lymphoma. 25: 532-535
(12) J Nucl Med 2001; Segall GM. FDG PET Imaging in patients with lymphoma: A clinical perspective. 42: 609-610 (No abstract available)
(13) J Cancer Res Clin Oncol 2000; Ilknur AK, et al. Positron emission tomography with 2-[18F] fluoro-2-deoxy-D-glucose in oncology: Part II: The clinical value in detecting and staging primary tumours. 126: 560-574
(14) Blood 1998; Romer W, et al. Positron emission tomography in non-Hodgkin's lymphoma: Assesment of chemotherapy with fluorodeoxyglucose. 91: 4464-4471
(15) J Nucl Med 2001; Schoder H, et al. Effect of whole-body 18F-FDG PET imaging on clinical staging and management of patients with malignant lymphoma. 42: 1139-1143
(16) Radiol Clin N Am 2001; Delbeke D, Martin WH. Positron emission tomography in oncology. 39: 883-917
(17) J Nucl Med 2002; Kostakoglu L, et al. PET predicts prognosis after 1 cycle of chemotherapy in aggressive lymphoma and Hodgkin's disease. 43: 1018-1027
(18) J Nucl Med 2002; Lowe VJ, Wiseman GA. Assessment of lymphoma therapy using 18F-FDG PET. 43: 1028-1030 (No abstract available)
(19) J Nucl Med 2003; Kostakoglu L, Goldsmith SJ. 18F-FDG PET evaluation of the response to therapy for lymphoma and for breast, lung, and colorectal carcinoma. 44: 224-239
(20) Br J Haematol 2001; Naumann R, et al. Prognostic value of positron emission tomography in the evaluation of post-treatment residual mass in patients with Hodgkin's disease and non-Hodgkin's lymphoma. 115: 793-800
(21) Leukemia 2002; Becherer A, et al. Positron emission tomography with [18F]2-fluoro-D-2-deoxyglucose (FDG-PET) predicts relapse of malignant lymphoma after high-dose therapy with stem cell transplantation.16:260-7
(22) Oncol Rep 2002; Shen YY, et al. Comparison of 18F-fluoro-2-deoxyglucose positron emission tomography and gallium-67 citrate scintigraphy for detecting malignant lymphoma. 9:321-5
(23) Blood 2001; Weihrauch MR, et al. Thoracic positron emission tomography using 18F-fluorodeoxyglucose for the evaluation of residual mediastinal Hodgkin disease. 98:2930-4
(24) GE PET Masters Series Clinical PET; Delbeke D. Verbal communication. Sept 18-20, 2002J
(25) J Clin Oncol 2001; Spaepen K, et al. Prognostic value of positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose ([18F] FDG) after first-line chemotherapy in non-Hodgkin's lymphoma: is [18F] FDG-PET a valid alternative to conventional diagnostic methods? 19: 414-419
(26) Radiology 2003; Bar-Shalom R, et al. Camera-based FDG PET and 67Ga SPECT in evaluation of lymphoma: comparative study. 227: 353-360
(27) J Nucl Med 2003; Rini JN, et al. 18F-FDG PET versus CT for evaluating the spleen during intial staging of lymphoma. 44: 1072-1074
(28) J Nucl Med 2003; Guay C, et al. Prognostic value of PET using 18F-FDG in Hodgkin's disease for posttreatment evaluation. 44: 1225-1231
(29) Radiology 2004; Rohren EM, et al. Clinical applications of PET in oncology. 231: 305-332
(30) Radiology 2004; Schaefer NG, et al. Non-hodgkins lymphoma and Hodgkin disease: coregistered FDG PET and CT at staging and restaging- do we need contrast-enhanced CT? 232: 823-829
(31) J Nucl Med 2004; Yamane T, et al. Decreased 18F-FDG uptake 1 day after initiation of chemotherapy for malignant lymphoma. 45: 1838-1842
(32) Radiol Clin N Am 2004; Kumar R, et al. Utility of fluorodeoxyglucose-PET imaging in the management of patient's with Hodgkin's and non-Hodgkin's lymphoma. 42: 1083-1100
(33) J Nucl Med 2005; Pakos EE, et al. 18F-FDG PET for evaluation of bone marrow infiltration in staging lymphoma: a meta-analysis. 46: 958-963
(34) Radiology 2005; Tatsumi M, et al. Direct comparison of FDG PET and CT findings in patients with lymphoma: initial experience. 237: 1038-1045
(35) J Nucl Med 2006; Jhanwar Y, Straus DJ. The role of PET in lymphoma. 47: 1326-1334
(36) J Nucl Med 2006; Rodriguez-Vigil B, et al. PET/CT in lymphoma: prospective study of enhanced full-dose PET/CT versus unenhanced low-dose PET/CT. 47: 1643-1648
(37) J Nucl Med 2007; Kasamon YL, et al. Integrating PET and PET/CT into risk-adapted therapy of lymphoma. 48: 19S-27S
(38) Radiology 2007; Schaefer NG, et al. Hodgkin disease: diagnostic value of FDG PET/CT after first-line therapy- is biopsy of FDG-avid lesions still needed? 244: 257-262
(39) J Nucl Med 2007; Weber WA. 18F-FDG PET in non-Hodgkin's lymphoma: qualitative or quantitative? 48: 1580-1582
(40) Radiographics 2006; Blake MA, et al. Pearls and pitfalls in interpretation of abdominal and pelvic PET-CT: 26: 1335-1353
(41) J Nucl Med 2008; Juwied ME. 18F-FDG PET as a routine test for posttherapy assessment of Hodgkin's disease and aggressive non-Hodgkin's lymphoma: where is the evidence? 49: 9-12
(42) J Nucl Med 2009; Jacene HA, et al. 18F-FDG PET/CT for monitoring the response of lymphoma to radioimmunotherapy. 50: 8-17
(43) J Nucl Med 2009; Ben-Haim S, Ell P. 18F-FDG PET and PET/CT in the evaluation of cancer treatment response. 50: 88-99
(44) AJR 2009; de Jong PA, et al. CT and 18F-FDG PET for noninvasive detection of splenic involvement in patients with malignant lymphoma. 192: 745-753
(45) J Nucl Med 2009; Itti E, et al. Prognostic value of interim 18F-FDG PET in patients with diffuse large B-cell lymphoma: SUV-based assessment at 4 cycles of chemotherapy. 50: 527-533
(46) AJR 2009; Otero HJ, et al. CT and PET/CT findings of T-cell lymphoma. 193: 349-358
(47) J Nucl Med 2010; Weiler-Sagie M, et al. 18F-FDG avidity in lymphoma readdressed: a study of 766 patients. 51: 25-30
(48) Radiology 2010; Storto G, et al. Assessment of metabolic response to radioimmunotherapy with 90Y-ibritumomab tiuxetan in patients with relapsed or refractory B-cell non-Hodgkin lymphoma. 254: 245-252
(49) Radiographics 2010; Paes FM, et al. FDG PET/CT of extranodal involvement in non-Hodgkin lymphoma and Hodgkin disease. 30: 269-291
(50) Radiographics 2010; Okada M, et al. FDG PE/CT versus CT, MR imaging, and 67Ga scintigraphy in the post-therapy evaluation of malignant lymphoma. 30: 939-957
(51) AJR 2010; Feeney J, et al. Characterization of T-cell lymphomas by FDG PET/CT. 195: 333-340
(52) J Nucl Med 2010; Cerci JJ, et al. 18F-FDG PET after 2 cycles of ABVD predicts event-free survival in early and advanced Hodgkin lymphoma. 51: 1337-1343
(53) J Nucl Med 2010; Itti E, et al. Improvement of early 18F-FDG PET interpretation in diffuse large B-cell lymphoma: importance of the reference background. 51: 1857-1862
(54) J Nucl Med 2013; Li YJ, et al. Prognostic value of interim and posttherapy 18F-FDG PET/CT in patients with mature T-ell and natural killer cell lymphomas. 54: 507-515
(55) J Nucl Med 2013; Moon SH, et al. The role of 18F-FDG PET/CT for initial staging of nasal type natural killer/T-cell lymphoma: a comparison with conventional staging methods. 54: 1039-1044
(56) J Nucl Med 2013; Kostakoglu L, Gallamini A. Interm 18F-FDG PET in Hodgkin lymphoma: would PET-adapted clinical trials lead to a paradigm shift? 54: 1082-1093
(57) J Nucl Med 2013; Berthet L, et al. In newly diagnosed diffuse large B-cell lymphoma, determination of bone marrow involvement with 18F-FDG PET/CT provides better diagnostic performance and prognostic stratification than does biopsy. 54: 1244-1250
(58) J Nucl Med 2014; Rossi C, et al. Interim 18F-FDG PET SUVmax reduction is superior to visual analysis in predicting outcome early in Hodgkin lymphoma patients. 55: 569-573
(59) J Nucl Med 2014; CArr R, et al. Prospective international cohort study demonstrates inability of interim PET to predict treatment failure in diffuse large B-cell lymphoma. 55: 1936-1944
(60) J Nucl Med 2015; Wondergem MJ, et al. 18F-FDG or 3'-Deoxy-3'-18F-fluorothymidine to detect transformation of follicular lymphoma. 56: 216-221
(62) AJR 2015; Heacock L, et al. PET/MRI for the evaluation of patients with lymphoma: initial observations. 204: 842-848
(63) J Nucl Med 2015; Jamar F, Lhommel R. Interim 18F-FDG PET in diffuse large B-cell lymphoma: emerging Worldwide? 56: 655-656
(64) Radiology 2015; Johnson SA, et al. Imaging for staging and response assessment in lymphoma. 276: 323-338
(65) Radiology 2015; Ulaner GA, et al. Prognostic value of FDG PET/CT before allogeneic and autologous stem cell transplantation for aggressive lymphoma. 277: 518-526
(66) Radiographics 2016; Ziai P, et al. Role of optimal quantification of FDG PET imaging in the clinical practice of radiology. 36: 481-496
(67) AJR 2017; Kulkarni NM, et al. Imaging for oncologic response assessment in lymphoma. 208: 18-31
(68) J Nucl Med 2017; Moghbel MC, et al. Response assessment criteria and their applications in lymphoma: part 2. 58: 13-22
(69) J Nucl Med 2017; Zwarthoed C, et al. Prognostic value of bone marrow tracer uptake pattern in baseline PET scans in Hodgkin lymphoma: results from an international collaborative study. 58: 1249-1254
(70) J Nucl Med 2017; Barrington SF, Johnson WM. 18F-FDG PET/CT in lymphoma: has imaging-directed personalized medicine become a reality? 58: 1539-1544
(71) J Nucl Med 2018; Dercle L, et al. 18F-FDG PET and CT scans detect new imaging patterns of response and progression in patients with Hodgkin lymphoma treated by anti-programmed death 1 immune checkpoint inhibitor. 59: 15-24
(72) J Nucl Med 2018; Chantepie S, et al. 18F-fludarabine PET for lymphoma imaging: first-in-humans study on DLBCL and CLL patients. 59: 1380-1385
(73) Radiology 2019; Metser U, et al. Effect of PET/CT on the management and outcomes of participants with Hodgkin and aggressive Non-Hodgkin lymphoma: A multicenter registry. 290: 488-495
(74) Radiology 2019; Scott JA. PET in lymphoma: making a difference in patients' lives. 290: 496-497
(75) AJR 2019; Sedig LK, et al. Do Deauville scores improve the clinical utility of end-of-therapy FDG PET scans for pediatric Hodgkin lymphoma. 212: 456-460
(76) Radiographics 2020; Sharma B, et al. Breast implant-associated anaplastic large cell lymphoma: review and multiparametric imaging paradigms. 40: 609-628
(77) J Nucl Med 2020;Gyorke T, et al. Combined visual and semiquantitative evaluation improves outcome prediction by early midtreatment 18F-FDG PET in diffuse large B-cell lymphoma. 61: 999-1005
(78) AJR 2020; Banks KP, et al. It's about quality, not quantity: qualitative FDG PET/CT criteria for therapy response assessment in clinical practice. 215: 313-324
(79) J Nucl Med 2021; Rekowski J, et al. Interim PET evaluation in diffuse large B-cell lymphoma using published recommendations: comparison of the Deauville 5-point scale and the ΔSUVmax method. 62: 37-42
(80) J Nucl Med 2021; Kurch L, et al. Interim PET in diffuse large B-cell lymphoma. 62: 1068-1074
(81) Radiographics 2022; Franquet T, et al. Human oncoviruses and thoracic tumors: understanding the imaging findings. 42: 644-660
(82) J Nucl Med 2022; Burggraaff CN, et al. 18F-FDG PET improves baseline clinical predictors of response in diffuse large B-cell lymphoma
(83) J Nucl Med 2023; Ricard F, et al. Application of the Lugano classification for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the PRoLoG consensus initiative (part 1- clinical). 64: 102-108
(84) J Nucl Med 2023; Michaud L, et al. Prognostic value of 18F-FDG PET/CT in diffuse large B-cell lymphoma treated with a risk-adpated immunotherapy regimen. 64: 536-541
(85) Radiographics 2023; Parihar AS, et al. FDG PET/CT-based response assessment in malignancies. 43: e220122
(86) J Nucl Med 2023; Chen X, et al. Fibroblast activation protein and glycolysis in lymphoma diagnosis: comparison of 68Ga-FAPI PET/CT and 18F-FDG PET/CT. 64: 1399-1405
(87) Radiology 2023; Mulholland N, et al. Chimeric antigen receptor T-cell therapy and imaging applications for large B-cell lymphoma. 307(5): e221362
(88) J Nucl Med 2024; Hirmas N, et al. Diagnostic accuracy of 68Ga-FAPI versus 18F-FDG PET in patients with various malignancies. 65: 372-378