J Clin Oncol 1996 Aug;14(8):2295-305
Clinical utility of external immunoscintigraphy with the IMMU-4
technetium-99m Fab' antibody fragment in patients undergoing surgery for carcinoma of the
colon and rectum: results of a pivotal, phase III trial. The Immunomedics Study Group.
Moffat FL Jr, Pinsky CM, Hammershaimb L, Petrelli NJ, Patt YZ, Whaley FS, Goldenberg DM.
PURPOSE: To assess the performance and the potential clinical impact of a new antibody
imaging agent, CEA-Scan (Immunomedics Inc, Morris Plains, NJ), in 210 presurgical patients
with advanced recurrent or metastatic colorectal carcinomas. METHODS: CEA-Scan, an
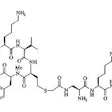
anti-carcinoembryonic antigen (CEA) Fab antibody fragment labeled with
technetium-99m-pertechnetate (99mTc), was injected intravenously (IV), and external
scintigraphy was performed 2 to 5 and 18 to 24 hours later. Imaging with conventional
diagnostic modalities (CDM) was also performed, and findings were confirmed by surgery and
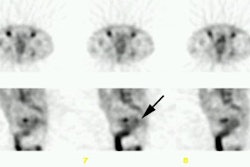
histology. RESULTS: The sensitivity of CEA-Scan was superior to that of CDM in the
extrahepatic abdomen (55% v 32%; P = .007) and pelvis (69% v 48%; P = .005), and CEA-Scan
findings complemented those of CDM in the liver. Among 122 patients with known disease,
the positive predictive value was significantly higher when both modalities were positive
(98%) compared with each alone (68% to 70%), potentially obviating the need for histologic
confirmation when both tests are positive. Imaging accuracy also was significantly
improved by adding CEA-Scan to CDM. In 88 patients with occult cancer, imaging accuracy
was enhanced significantly by CEA-Scan combined with CDM (61% v 33%). Potential clinical
benefit from CEA-Scan was demonstrated in 89 of 210 patients. Only two patients developed
human antimouse antibodies (HAMA) to CEA-Scan after a single injection, and none of 19
assessable patients after two injections. CONCLUSION: CEA-Scan affords high-quality,
same-day imaging, uses an inexpensive and readily available radio-nuclide, adds clinically
significant information in assessing extent and location of disease in colorectal cancer
patients, and only rarely induces a HAMA response.
Publication Types:
- Clinical trial