Renal Vascular Hypertension
Clinical Presentation
Renal vascular hypertension (RVH) is believed to be the etiology
of hypertension (HTN) in only 1 to 4% of hypertensive patients. It
is the cause of about 5% of all cases of childhood hypertension.
A slowly-developing renal artery stenosis allows the opening of
collaterals and may result in a severely ischemic kidney with poor
function that survives on this collateral flow, but produces large
quantities of renin to sustain renovascular hypertension. An acute
renal artery obstruction usually leads to partial or complete
infarction and atrophy, without hypersecretion of renin.
Risk factors for renovascular hypertension include: a diastolic blood pressure greater than 95 mm Hg in a patient that is refractory to three antihypertensive medications, accelerated or abrupt onset of hypertension, hypertension under the age of 30 years, sudden loss of previous hypertension control, impairment/worsening in renal function following captopril administration (or other angiotensin converting enzyme inhibitors), or an abdominal/flank bruit [23].
A normal renal vein renin level is usually less than about 0.24. A level greater than 0.48 is considered consistent with renovascular HTN (Sensitivity 56-72%, Specificity 30-90%). Unfortunately, up to 65% of patients with non-lateralizing renal vein renin levels will demonstrate improved HTN control after revascularization.
Hemodynamic Significance
Renal Artery Stenosis is considered hemodynamically significant where there is:
- Renin ratio of the affected to the unaffected side of greater than 1.5:1
- Greater than 50% diameter stenosis of the renal artery with post-stenotic dilatation.
- Pressure gradient across stenosis of over 40 mm Hg
As with renal vein renins, specificity in predicting response to therapy is poor.
Etiology of Renovascular Hypertension
Common causes of unilateral or bilateral main or branch renal artery stenosis include:
- Atherosclerosis: Atherosclerosis accounts for most cases of RVH (70-90% of cases [22,23]). Atherosclerotic renal artery stenosis tends to involve the origin (proximal 2 cm) and major bifurcations of the renal artery (although medium sized vessel disease within the kidney may be seen). The involvement may be segmental. The stenosis of the renal artery tends to be progressive and will eventually lead to occlusion of the vessel.
- Fibromuscular
Dysplasia: FMD is the second most common cause of
RAS [24]. It accounts for 10-30% of cases of RVH [22].
Predominantly affects young female patients (F:M ratio is 9:1)
[22,24]. The renal artery is the most commonly affected vessle
in FMD and is involved in 75% of cases (the internal carotid
artery is the next most commonly involved) [24]. FMD-related
stenoses tend to occur in the middle to distal portion of the
renal artery and bilateral involvement occurs in two-thirds of
patients [24]. Up to 10% of cases have associated renal artery
aneurysms [24]. PTCA is the treatment of choice and has an
initial success rate of 90% in these patients and a 75% patency
rate after 2 years. Endolumenal stent placement or surgical
bypass are reserved for cases of treatment failure or
complications such as dissection [24].
- Arteritis:
- Polyarteritis Nodosa: The renal vasculature is the most common site of involvement [24]. Patients present with nonspecific symptoms such as fever, weight loss, and polyarthragia [24]. CT can show multiple small bilateral renal infarcts of various ages distributed among the interlobar and arcuate arteries [24]. Angiography will show multiple 2-3 mm microaneurysms [24].
- Takayasu's Arteritis, XRT, or Syphilitic Arteritis
- Aortic Dissection: A dissection of the descending aorta will typically affect the artery to the left kidney.
- Neurofibromatosis: Renal artery stenosis is the most
common vascular abnormality in patients with NF1 and the most
common cause of hypertension in pediatric NF1 patients [25].Patients
develop a renal artery stenosis due to an underlying mesodermal
dysplasia [25]. The stenosis occurs at the ostia and can be
unilateralor bilateral [25].
- Trauma
- Umbilical Artery Catheterization: The placement of an umbilical artery catheter may be complicated by aortic or renal artery thrombosis, which can produce renal ischemia and RVH due to impingement of the thrombus on the renal artery ostium. Therapy with angiotensin converting enzyme inhibition is generally successful in these neonates, but may precipitate renal failure if the ischemia is bilateral or if there is only one functioning kidney.
- Extrinsic compression of the renal artery or branches
- Renal infarction (total or partial)
- Renal artery aneurysm
- Tumors of the juxtaglomerular apparatus
- Hydronephrosis (Very rarely)
- Other renal abnormalities: Infarction, post-pyelonephritis cortical scarring, and post-traumatic injuries may also result in hypertension.
Pathophysiology of Renovascular Hypertension
The pathogenesis relates to a decrease in the perfusion pressure distal to the stenosis resulting in a reduction in the transglomerular pressure gradient that produces a decrease in the glomerular filtrate (this leads to a decrease in sodium delivery to the distal tubule) [23]. In renal artery stenosis, transcapillary pressures to drive glomerular filtration are maintained by a preferential increase in the efferent arteriolar resistance behind the glomerulus. This increased efferent arteriolar resistance is maintained by angiotensin-II (which is produced in response to increased secretion of renin from the involved kidney). Angiotensin II also stimulates aldosterone secretion from the adrenal cortex which contributes to sodium and fluid retention (producing plasma volume expansion to maintain physiologic renal perfusion by increasing blood pressure [22]). When a critical level of renal artery stenosis is reached (about 60-70% of the lumen) baroreceptors in the kidney sense the decreased blood pressure in the afferent arteriole, which results in increased renin release from the juxtaglomerular apparatus - renin cleaves angiotensin I from angiotensinogen. This results in increased production of angiotensin I. Angiotensin I is then converted in the periphery and the kidney by the action of angiotensin converting enzyme into angiotensin II [23]. Angiotensin II is a potent vasoconstrictor which preferentially constricts the efferent arteriole of the glomerulus, raising the transglomerular pressure gradient and functioning to maintain GFR [23].
Captopril, an angiotensin converting enzyme inhibitor, acts to block the conversion of angiotensin I to angiotensin-II, which induces efferent arteriole vasodilatation. ACE inhibitors also inhibit kininase II - a peptidase that inactivates bradykinin [23]. Bradykinin then accumulates and is a potent vasodilator causing selective efferent arteriolar dilatation [23]. The effect of bradykinin is probably quite important since only 60% of angiotensin II is synthesized by ACE-dependent pathways in the human renal cortex and neither acute nor chronic ACE inhibition completely eliminates angiotensin II from the plasma [23]. Thus, post-capillary resistance is decreased and the filtration pressure and GFR fall if the kidney has significant renal artery stenosis. Despite this decline in GFR, effective renal plasma flow does not change significantly. Since the tubular cells retain their functional capacity the kidney will continue to accumulate tubular agents such as MAG3. However, because of insufficient urine production, the tracer is not cleared and remains in the renal cortex [23]. This leads to a prolonged parenchymal transit of the renal tubular tracers.
In patients with a solitary kidney or bilateral critical RAS/RVH, captopril may precipitate acute renal failure, but this effect is usually only transient.
Afferent arteriolar tone is predominantly regulated by calcium entry into smooth muscle cells and may be inhibited by calcium channel blockers (nifedipine and verapamil). Patients taking calcium antagonists may have false positive exams characterized by bilateral symmetric decreased renal function [1].
Treatment of RVH due to Renal Artery Stenosis (RAS)
Percutaneous Transluminal Angioplasty in the Treatment of RAS:
- Atherosclerosis: Following percutaneous translumenal angioplasty for atherosclerotic renal artery stensosis, blood pressure returns to normal in about 10% of patients, improves in 50%, and does not change in 40%. The treatment has a higher success rate (cured or improved) in patients with non-osteal stenoses (88%) than for osteal stenoses (40%).
- Fibromuscular Dysplasia: Blood pressure returned to normal in 60%, improved in 20%, and did not change in 20% after PTCA. Grouping PTCA for both FMD and ASPVD Losinno et al. found a technical and clinical success rate of approximately 80% at 10 years post-procedure. Renal function improved (as evidenced by an improvement in creatinine) in 50% of patients with bilateral stenoses or stenosis to a single functioning kidney following PTCA. [2]
Radiographic Findings in Renal Artery Stenosis
On IVP the affected kidney is usually smooth and small (greater
than 2cm size difference from the opposite kidney). RAS is one of
the causes of global tissue loss with a smooth contour. There is
an initially decreased nephrogram which becomes increasingly dense
over time with delayed washout and delayed opacification of the
collecting system. Pelvic/ureteral notching may be identified due
to the presence of dilated collateral vessels. Arterial
calcification may be seen on the scout film.
On US, diagnostic criteria can be divided into direct and
indirect signs [24]. Direct signs include a PSV greater than 200
cm/sec; a renal artery PSV to pre-renal abdominal aortic PSV ratio
higher than 3.5:1; a lack of Doppler US signal indicative of
occlusion; and the presence of color artifacts such as aliasing
related to turbulence [24]. Indirect signs include a spectral
waveform with a delayed and blunted systolic upstroke (pulsus
parvus et tardus) which correlates with an acceleration index
lower than 300 cm/sec, an acceleration time longer than 70 msec,
and loss of an early systolic peak [24]. For RAS, the presence of
a pulsus parvus et tardus waveform has a specificity of 96% and a
PPV of 92%, but the sensitivity is only 43% [24]. A resistive
index of higher than 0.80 has been suggested as an indicator of
poor therapeutic response to revascularization [24].
CT and MR angiography are excellent tests to detect renal artery stenosis (accuracy of CT is greater than 90% [24]), but it cannot provide information regarding outcome following angioplasty.
Angiography can also demonstrate the presence of RAS, but gives no physiologic information regarding presence of RVH. Findings include a delayed nephrogram and a stenosis with post-stenotic dilatation. Renal vein sampling can detect the increased renin levels which localize to the involved side in the setting of renovascular hypertension.
Nuclear Medicine Examination of Renovascular Hypertension
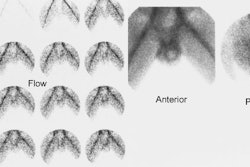
The scintigraphic evaluation of renovascular hypertension is best performed in patients with a moderate to high risk for condition (Risk factors include: a diastolic blood pressure greater than 95 mm Hg in a patient that is refractory to 3 anti-hypertensive medications (RVH observed in 15-30% of this patient population [22]); accelerated or abrupt onset of hypertension; sudden loss of previous hypertension control; impairment in renal function following captopril administration, angiotensin converting enzyme inhibitors, or angiotensin receptor blockers; or an abdominal bruit [22]. In patients with renovascular hypertension, the administration of an angiotensin converting enzyme inhibitor will induce decreased tracer uptake or delayed excretion with cortical retention (or both) on the affected side [17]. Time-activity curves should reveal these alterations in renal function [17]. Interobserver agreement regarding exam interpretation is generally satisfactory.
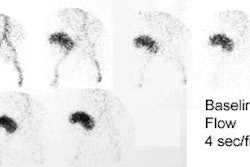
Patients are scanned using Tc-MAG3 before and after the administration of captopril (an angiotensin converting enzyme inhibitor). The examination can be performed as a one day or two day protocol. For the one day exam, a low dose (1 mCi) baseline exam is performed first and followed by a high dose (9 mCi) post-ACE inhibitor study. If using a two day protocol, the ACE exam (10 mCi MAG3) should be performed first- if this is normal- a baseline study is not required.
ACE inhibition renography is a test to detect a functionally
significant RAS; it is not a test to detect the presence of an
anatomic RAS [23]. A positive ACE inhibition scintigraphy exam
indicates that RVH is present and implies the existence of
hemodynamically significant renal artery stenosis (greater than 60
to 75% of the lumen). For renal artery stenosis > 50% using
Tc-MAG3: [4] Sensitivity: 90%, Specificity: 91%, Positive
predictive value: 70%, Negative predictive value: 97%. The main
benefit of the nuclear renovascular hypertension exam is to
determine which patients can be expected to demonstrate
improvement in blood pressure control following revascularization.
The positive predictive value for clinical improvement in
hypertension following revascularization varies between 51% to
100% (mean 85%) [13,20]. Although the exam is generally accurate,
it can often be non-diagnostic/less accurate in up to 50% of
azotemic patients or in patients with a small, poorly functioning
kidney [23].
Examination Techniques
Medications: Concurrent anti-hypertensive medications can affect the accuracy of the examination [21]. The sensitivity has been shown to decrease from 98% in patients on no medications, to 87% when they are on a diuretic, and 75% if the patient is on an ACE inhibitor [5]. Prior to their baseline exam, patients should be off ACE inhibitors for at least 48 hours (generally for 4 days for Captopril and 7 days for longer acting agents depending on the half-life of the agent) [7]. Captopril, enalapril, and lisinopril have half-lives of about 48, 72, and 96 hours respectively. If needed, amlodipine or labetalol may be substituted [22]. Diuretics should be with-held for the 48 hours prior to the exam to prevent dehydration. Patients taking calcium antagonists may have false positive exams characterized by bilateral symmetric decreased renal function [6] and it is prefered that these agents also be discontinued prior to the exam [22]. There is no need to stop propranolol.
Angiotensin II receptor blockers such as Losartan (Sartans)
induce a dose dependent blockade of angiotensin II effects and are
generally better tolerated than ACE inhibitors [21]. Angiotensin
II receptor blockers may have an effect comparable to chronic ACE
inhibition and this agent should also be discontinued for 4-7 days
prior to the exam [18,22]. However, other authors have not found
chronic sartan therapy to affect the ACE exam (sensitivity 92% in
a small number of patients studied) [21,23].
Diclofenac, a nonsterodial antiinflammatory drug, blocks the
production of prostaglandins and has been shown to inhibit
spontaneous ureteric contraction, prolong the transit time, and
delay time to peak height of the renogram curve of MAG3 in healthy
individuals [23].
Patient preparation: It
is advisable that the patient have a heparin lock in place- this
will permit venous access should the patient experience
significant hypotension during the exam. Either Captopril or
Enalaprilat can be used as the ACE inhibitor. Captopril (25-50 mg
crushed) is given orally with 250-500 mL of water 60-90 minutes
prior to start of exam. The dose of Captopril in children is 1
mg/kg up to 50 mg [verbal communication Dr. Madj, Childrens
Hospital, Washington, D.C.]. Since the presence of food in the GI
tract can reduce the absorption of captopril by 30-40%, patients
should avoid solid food for 4 hours prior to the exam [23]. Peak
blood levels occur approximately 60 minutes after oral
administration and therefore the exam should be delayed for 60-90
minutes after captorpil administration [23].
Enalaprilat .04mg/kg (Maximum 2.5 mg) is given via slow (over 5 minutes) I.V. infusion 10-15 minutes prior to the start of the exam [23]. Enalaprilat has a longer duration of action than captopril. Another advantage of enalaprilat is that it is administered intravenously and its effect is not affected by a variable rate of absorption through the GI tract (false negative exams can occur due to delayed gastric emptying or poor absorption of Captopril) [23].
The blood pressure is monitored every 15 minutes after oral Captopril, or every 5 minutes after I.V. Enalaprilat. It is advisable NOT to proceed with the examination when an adult patient on therapy has a resting systolic pressure less than 140 mm Hg or when an infant has a mean arterial pressure below 65 mm Hg. In these patients, ACE inhibition may result in marked hypotension. If blood pressure falls below safe levels (about 30% of the original systolic value), the rate of I.V. fluid infusion should be increased. Patients should not be permitted to leave the department unless their blood pressure is at least 70% of baseline and they are asymptomatic. A statement to this effect should be included in the report.
Diuresis: Furosemide 40mg (1mg/kg) may be given at the start of the exam to minimize the likelihood of tracer retention in the collecting system, but is not mandatory (particularly if the patients are well hydrated for the exam) [23]. Oliguria is not uncommon following ACE administration due to the peripheral action of the agent. This oliguria may result in retention of radiotracer in the collecting system and the associated renal cortex generating a false-positive difference from the baseline exam. If using lasix, there is a greater risk of hypotension and an I.V. line can be used to provide supplemental hydration. A full urinary bladder may also slow clearance or predispose to reflux; bladder catheterization may be needed in selected cases.
Adequate hydration is essential to maintain renal perfusion. Oral
hydration with 10 ml/kg is given prior to the start of the exam.
An I.V. line is maintained throughout the exam and 500 ml of
Normal Saline is infused at 2-4 ml/min.
Dose infiltration will result in errors in the camera-based
measurements of clearance and can lead to bilaterally abnormal
renogram curves due to continued absorption of the infiltrated
dose [23].
Scintigraphic Findings
Consensus panels recommend that the test be interpreted as high, low, or intermediate (indeterminate) probability for RVH [23]. A normal examination indicates a very low probability for renovascular hypertension (under 10%) [13]. A positive examination confirms the presence of an activated renin-angiotensin mechanism. This is most commonly secondary to renal artery stenosis, but other disorders such as vasculitis and scleroderma may also activate this system. A small, poorly functioning kidney (under 30% uptake) with a time to maximum activity over two minutes that shows no change on the ACE exam is sometimes considered indeterminate for RVH. Bilateral symmteric abnormalities (such as cortical retention of the agent) also indicate an intermediate probability of RVH [13]. However, some individuals consider bilateral symmetric cortical retention on the ACE exam to most likely be a false positive due to hypotension, dehydration, or calcium channel blocker use.
For tubular agents (MAG3, OIH), findings indicative of a high probability of renal vascular hypertension include:
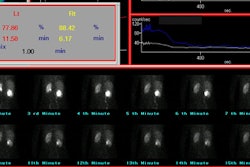
- Prolonged Cortical Retention (increased parenchymal transit time): Reduced urine flow causes delayed and diminished washout of radiotracer into the collecting system which will quantitatively appear as an increase in cortical retention [19]. Normally, there should be less than 30% of the peak activity retained in the renal parenchyma at 20 minutes of the exam (in fact, with MAG3, there is generally less than 20% retained activity at this time). On the captopril exam, if the retained cortical activity at 20 minutes is less than 30%, this virtually excludes RVH and a baseline exam is not necessary. An increase in residual cortical activity at 20 minutes of at least 15 percentage points from the baseline exam is considered diagnostic of RVH (and a 70-90% RAS). A 5-10% increase in retained cortical activity may be considered suspicious and warrants further evaluation. [8,9]
- Split renal function decrease: A unilateral decrease in renal function of more than 5 percentage points (5%) or displacement outside the normal range (44-56%) after Captopril administration is considered suspicious for RVH. Other authors quote a decrease of greater than 10% change in relative renal function as high probability for RVH [13,19,23]. A change in renal function is not commonly seen, but it is very significant if it occurs.
- Time to peak cortical activity: An increase in the time to peak cortical activity of more than 5 minutes after Captopril compared to baseline is considered very suspicious for RVH. Other authors quote an increase of 2 minutes in time to peak activity [19,23] or a change in time to peak activity of greater than 40% as high probability for RVH [13]. One point to remember is that a change in time to peak activity from 5 to 7 minutes is more significant than a change from 15 to 17 minutes [23].
- Rising Baseline Renogram: Cases of severe stenosis (over 95%) have been seen with a rising baseline MAG3 renogram and no further deterioration after ACE inhibition (Nuclear Medicine Annual, 92, p.175). In these patients the renin-angiotensin compensation is no longer adequate to maintain renal perfusion. Other conditions may also produce a rising baseline exam, including ATN, drug toxicity, interstitial or glomerular nephropathies, complete obstruction, and renal vein thrombosis, so specificity of this finding is suboptimal (50-75%).
- Non-visualization of the kidney: This can be seen in complete obstruction of the renal artery without major collaterals.
Diagnostic Criteria Based upon Evaluation of the Renogram Curve:
- Grade 0 - Normal
- Grade 1 - Mild delay in upslope with maximal activity seen between 6 and 11 minutes, or delay in the excretory phase.
- Grade 2 - Delay in the upslope and Tmax, but an excretory phase is still seen.
- Grade 3 - As above, but without an excretory phase
- Grade 4 - Renal failure with measureable uptake
- Grade 5 - Renal Failure without measureable function.
Positive Exam: Deterioration in grade following captopril administration is deemed high probability of renal artery stenosis, no change in grade (except grade 0) as indeterminate, and improvement in grade as low probability of renal vascular hypertension. Some consider no change in grade for a grade 1 curve as low probability for RVH.
Tc-DTPA (Glomerular Agent) in the Evaluation of RVH
A decrease in the cortical uptake between 2-3 minutes of the examination is expected to occur in RVH. This can be seen as a flattening of the time-activity curve with a prolonged time to peak renal activity (a change of at least 2 minutes to peak activity or over 11 minutes on either the pre- or post-captopril exam), or expressed as a change in the split or absolute renal functions at these times- a unilateral decrease in differential function of at least 5-9% (suspicious/intermediate) or greater than 10% (high probability). Another criteria is the GFR ratio between the kidneys which will become greater than 1.5 on the post-captopril exam. In severe stenosis (over 95%), the affected kidney will be hypofunctioning and a captopril effect may not be seen. A diuretic is not necessary for this examination because RVH criteria are based upon the rate of accumulation of the tracer, NOT on excretion. However, marked unilateral retention, although uncommon, represents a high probability study.
Prognostic Value of the Captopril Renal Scan:
A positive captopril examination is a strong predictor of surgically correctable renovascular hypertension (97%). Approximately 85% to 90% of patients with an abnormal exam will demonstrate improvement in blood pressure following intervention [10,13]. No change in renal function after captopril in a patient with known RAS predicts a poor response to angioplasty or surgery; however, about 30% of these patients will still demonstrate some improvement following intervention.
In cases of severe RAS (greater than 90%) the compensatory function of the renin-angiotensin system may be incomplete, and the baseline study in these patients can be abnormal. These kidneys may be atrophic and hypofunctioning. A detectable change may not be appreciated in these patients following the administration of captopril.
False positive exams: Patients who develop marked hypotension (Systolic BP < 100 or a blood pressure drop of greater than 20 mmHg) following ACE inhibitor administration may have a false-positive examination. Frequently this effect is bilateral [14]. Patients with low-output congestive heart failure may have false-positive examinations. Renal arteriolar vasculitis may also produce a false-positive exam.
Captopril Scintigraphy in Patients with Hypertension and Chronic Renal Failure
Hypertension is an invariable consequence of the loss of renal function in patients with chronic renal failure. ACE inhibitors are now being used with increasing frequency in patients with hypertension and renal failure since there is evidence to suggest that they may be renoprotective. However, in clinical situations where the renin-angiotensin system is activated, ACE inhibitors can have a detrimental effect on renal function.
In these patients, a normal captopril exam may indicate a beneficial effect of ACE inhibition on renal function. At a minimum it indicates that the danger of treating the patient's hypertension with an ACE inhibitor is minimal. If the study is abnormal, the renin-angiotensin system is activated and ACE inhibition therapy is contraindicated. Once GFR drops below 10 ml/min. or split function becomes 10% or less, Tc-MAG3 captopril scintigraphy in these patients is generally unreliable [7,10]. Other authors feel that Captopril scintigraphy can still adequately detect renal artery stenosis in renal failure patients [12].
REFERENCES:
(1) J Nucl Med 1998; Claveau-Tremblay R, et al. False-positive captopril renography in patients taking calcium antagonists. 39: 1621-1626
(2) AJR 1994; Apr: 853
(3) J Nucl Med 1996; Taylor A, et al. Consensus report on ACE inhibitor renography for detecting renovascular hypertension. Radionuclides in Nephrourology Group. Consensus Group on ACEI Renography. 37(11):1876-82. No abstract available.
(4) Dondi et al, EJNM, 1990
(5) HTN 1991; 18; 289-98
(6) J Nucl Med 1998; Claveau-Tremblay R, et al. False-positive captopril renography in patients taking calcium antagonists. 39: 1621-1626
(7) J Nucl Med 1994; Datseris IE, et al. Captopril renal scintigraphy in patients with hypertension and chronic renal failure. 35(2):251-4.
(8) Radiol Clin North Am 1993; Sfakianakis GN, et al. Diagnosis of renovascular hypertension with ACE inhibition scintigraphy.31(4):831-48. Review. No abstract available.
(9) Nuclear Medicine Annual 1992; Sfakianakis GN, et al. Scintigraphy in acquired renal disorders. Ed. Freeman LM. Raven Press, Ltd. New York. 157-224
(10) J Nucl Med 1994; Blaufox MD. Should the role of captopril renography extend to the evaluation of chronic renal disease? 35(2):254-6. No abstract available.
(12) J Nucl Med 1999; Fernandez P, et al. Value of captopril renal scinitgraphy in hypertensive patients with renal failure. 40: 412-417
(13) Radiographics 2000; Soulez G, et al. Imaging of renovascular hypertension: Respective values of renal scintigraphy, renal doppler US, and MR angiography. 20: 1355-1368
(14) J Nucl Med 1999; Stavropoulos SW, et al. Hypotensive response to captopril: A potential pitfall of scintigraphic assessment for renal artery stenosis. 40: 406-411
(15) J Nucl Med 1991; Dondi M, et al. Use of technetium-99m-MAG3 for renal scintigraphy after angiotensin-converting enzyme inhibition. 32: 424-428
(16) Semin Nucl Med 1992; Nally JV, Black HR. State-of-the-art review: Captopril renography- pathophysiological considerations and clinical observations. 22: 85-97
(17) J Nucl Med 2002; Krijnen P, et al. Interobserver agreement on captopril renography for assessing renal vascular hypertension. 43: 330-337
(18) Clinical Nuclear Medicine Review Course 2002; Taylor A. Renal scinitgraphy. 3-24.
(19) Radiographics 2002; Saremi F, et al. Pharmacologic interventions in nuclear radiology: indications, imaging protocols, and clinical results. 22: 477-490
(20) AJR 2003; Soulez G, et al. Prediction of clinical response after renal angioplasty: respective value of renal doppler somography and scintigraphy. 181: 1029-1035
(21) J Nucl Med 2003; Picciotto G, et al. Reliability of captopril renography in patients under chronic therapy with angiotensin II (AT1) receptor agonists. 44: 1574-1581
(22) J Nucl Med 2006; Boubaker A, et al. Radionuclide
investigations of the urinary tract in the era of multimodality
imaging. 47: 1819-1836
(23) J Nucl Med 2014; Taylor AT. Radionuclides in nephrology,
part 2: pitfalls and diagnostic applications. 55: 786-798
(24) Radiographics 2017; Al-Katib S, et al. Radiologic assessment
of native renal vasculature: a multimodality review. 37: 136-156