PET in Infection Imaging:
General:
PET imaging has several advantages over convention nuclear
infection imaging including tomographic scans with excellent
spatial resolution and same day imaging [11]. FDG PET appears to
be very good for the evaluation of suspected infection of
the musculoskeletal system. Activated inflammatory cells
demonstrate increased expression of glucose transporters- GLUT1
and GLUT3 [10,33]. The physiologic basis of FDG PET in
the identification of infection is likely related to the
respiratory burst that neutrophils and monocytes experience when
exposed to proinflammatory cytokines (e.g., granulocyte?macrophage
colony stimulating factor, interleukin-8, and
interleukin-6), with the resulting metabolization of
large amounts of glucose (ie: inflammatory cells at sites of
infection show increased glycolytic activity) [1,2]. Increased
splenic activity can be seen in patients with underlying
infections- presumably related to increased glucose utilization
[10]. However, FDG imaging cannot discriminate between and
infectious and an inflammatory process [38].
Antibiotic treatment does not appear to significantly impact on
the diagnostic accuracy of FDG PET imaging for evaluaiton of
infection, however, the SUVmax in sites of infection have been
shown to be slightly lower in these patients compared to untreated
patients (although not statistically significant) [48]. In animal
models, both hyperglycemia and hyperinsulinemia have been shown to
decrease FDG uptake at sites of infection and inflammation [15].
The major indications for 18F FDG
PET/CT in infection and inflammation are sarcoidosis, peripheral
bone osteomyelitis (nonpostoperative, nondiabetic foot),
suspected spine infection (spondylodiskitis or vertebral
osteomyelitis, nonpostoperative), evaluation of FUO, evaluation
of metastatic infection and of high-risk patients with
bacteremia, and primary evaluation of vasculitides (such as
giant cell arteritis) [33].
It is presently unclear if FDG PET offers a
significant advantage over labeled WBC imaging for diabetic foot
infection, joint prosthesis infection, vascular prosthesis
infections, inflammatory bowel disease, or endocarditis [33].
False positive exams have been reported in up
to 10% of patients and are associated with chronic granulocytic
or reactive post surgical changes, inflammatory processes,
Charcot osteoarthropathy, fractures, and retroperitoneal
fibrosis [48].
18F- FDG-labeled white bloods have also be evaluated for use in infection imaging [9].
Metallic implants:
About 400,000 hip and knee arthoplasties are performed annually in the United States [7]. Infection after primary implantation occurs in approximately 1% of primary hip arthroplasties and 2% of primary knee arthroplasties [7]. The rate of infection following revision surgery is higher- about 3% for hip and 5% for knee replacements [7]. Approximately one-third of infections develop within 3 months of surgery, another third within 1 year, and the remainder more than 1 year after surgery [7]. FDG PET imaging has been used to evaluate for infected orthopedic prostheses and can assess for the presence of both acute and chronic infectious processes [1]. Advantages of FDG PET imaging over conventional imaging include: 1- FDG accumulates within the site of infection within 60 minutes which provides for rapid patient evaluation; 2- PET imaging has a much higher spatial resolution compared to single photon techniques which allows better distinction between soft-tissue and osseous infection; and 3- FDG PET imaging is less expensive than leukocyte-based labeling techniques [1].
When evaluating FDG PET/CT imaging for prosthetic infection, careful attention to the non-attenuation corrected images is recommended, because there can be false elevated activity on attenuation corrected images due to beam-hardening artifact on the CT images [49]. Initially, FDG PET imaging had shown great utility for the evaluation of patients with suspected orthopedic prosthesis infections [1]. Sensitivity of up to 100%, specificity of 88-93%, and accuracies of 97% had been reported with low interobserver variability [1]. Recently, lower sensitivities for infected hip prosthesis have been reported (as low as 22%)- with an overall accuracy of only 69% [6]. This is because aseptic loosening and synovitis can also produce a positive PET exam [6,7]. In aseptic loosening, a foreign body granulomatous reaction to polyethylene particles shed by the prosthesis, results in macrophage activation that can produce increased FDG accumulation (despite the absence of neutrophils [42]) [6]. Lower specificity has also been reported (between 9% to 44% depending on the criteria used to define infection) [7].
It is clear that the location of abnormal accumulation is an important element in image interpretation [11]. Intense FDG uptake along the bone-prosthesis interface should be considered positive for infection, mild uptake as suspect for loosening, and uptake only in the soft tissues as evidence of synovitis [11].
Unfortunately, regardless of how the images are interpreted, FDG PET imaging appears to be less accurate than 111In-labeled leukocyte/ 99mTc-sulfur colloid marrow imaging [7].
Scans performed earlier than 6 weeks following surgery may be falsely positive [1]. Other causes of false positive exams are sterile inflammation associated with prosthetic loosening and patient motion between emission and transmission scans [1]. FDG uptake around the head and neck of hip prosthesis can be seen commonly in non-infected prostheses [3], whereas increased tracer uptake along the interface between the bone and the prosthesis is suggestive of infection [4].
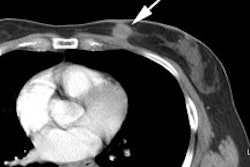
Vascular graft infection:
Vacular graft infection is an uncommon (0.5-5% of cases)
complication associated with a high morbidity and mortality [16].
As a rule, most infections occur within a few months of surgery
[16]. The clinical presentation is often non-specific [16]. CT and
MR imaging may demonstrate a surrounding abscess collection, but
the findings are often inconclusive (in one study CT had a
sensitivity of 64% [21]) [16].
Synthetic grafts are made either of Dacron or
polytetrafluroethylene (Gore-Tex) [36]. Dacron is used mainly in
large vessels such as with aortic or aortoiliac surgery, whereas
Gore-Tex is used for medium-sized vessels such as the femoral,
popliteal, and tibial arteries [36]. Synthetic grafts often induce
an aseptic foreign-body chronic low-grade inflammatory
reaction (mediated primarily by macrophages, fibroblasts,
and foreign-body giant cells) that can result in tracer uptake
along the graft [16,17,21,36]. In one study, high FDG accumulation
was identified in 75% of non-infected aortic vascular grafts
placed by open surgery (and in one of 4 grafts [25%] placed
endovascularly) [17]. In another study, diffuse FDG uptake was
found in 92% of non-infected vascular prostheses [32]. The uptake
is typically homogeneous, but can be heterogeneous, particularly
for Dacron grafts (heterogeneous uptake in a Gore-tex graft should
be interpreted with caution) [36]. In general, Dacron grafts also
demonstrate a higher level of tracer uptake compared to Gore-Tex
grafts and native vein grafts [36]. The uptake in vascular grafts
can persist for 10-20 years following surgery and does not change
over time of prosthetic grafts [17,36]. However, activity in
native vein grafts does decrease over time and persistent activity
in a native vein graft should raise the suspicion for infection
[36].
When associated with infection, the tracer uptake appears to be
more focal, eccentric, and more intense than background graft
activity [17]. For best results, PET imaging should be performed
at least 2 months following surgery to avoid false-positive
results [21].Initial studies suggested that PET/CT imaging could
aid in more definitive localization of the site of tracer uptake
permitting accurate differentiation of graft infection versus
adjacent soft tissue infection [16]. In one study, FDG PET/CT was
found to have a sensitivity of 93%, specificity of 91%, a PPV of
88%, and a NPV of 96% [16]. False positive results can occur when
the infection is adjacent to or surrounding the graft [16]. In the
early phases of healing during the first months following surgery,
FDG imaging may give false positive results [15]. .
Osteomyelitis:
Increased FDG uptake can be seen at sites of osteomyelitis [10]. FDG PET has been shown to be superior to 111In-labeled leukocyte scintigraphy for diagnosing chronic bacterial osteomyelitis and for establishing a source of fever of unknown origin [41]. However, false positive exams can occur at sites of acute fracture, in normal healing bone up to 4 months after surgery, and in inflammatory arthritis [10]. Mild FDG accumulation is also seen within the normal bone marrow [10].
Chronic osteomyelitis is typically the result of inadequately treated acute osteomyelitis or it may follow exogenous bacterial contamination related to trauma or surgery [15]. It is characterized by the presence of lymphocytic and plasma cell infiltrates [15]. FDG PET imaging has been shown to have very good accuracy for the evaluation of chronic osteomyelitis [15].
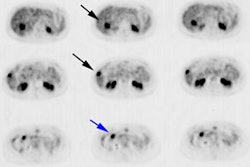
FDG PET imaging can be used for the diagnosis of diabetic foot
infections [8]. In diabetic patients, FDG might be at a
disadvantage for evaluation of infection, however, the effect of
hyperglycemia on FDG uptake at sites of infection and inflammation
is not well documented [8,21]. In at least one study, elevated
glucose levels did not seem to affect detection of sites of
infection [8].
FDG PET can be positive in 81 to 93% of diabetic foot
infections, with a specificity of 91% [8,21]. In a meta-analysis
FDG PET/CT had a reported sensitivity of 74% and a specificity of
91% in diagnosing osteomyelitis in diabetic foot ulcers [49].
Unfortunately, lower sensitivity (43%), specificity (67%), and
accuracy (54-62%) have also been reported [23].
Charcot joint typically demonstrates a low degree of of diffuse
uptake [49]. SUVmax values can
also aid in differentiating neuropathic joint from infection
[23]. In one study, the SUV max associated with osteomyelitis
(4.38 +/- 1.39) was higher than that of neuropathic joint (1.3 +/-
0.4) [23].
Precise localization of the site of uptake can be difficult on
conventional PET imaging [8]. The use of PET/CT imaging aids in
the accurate localization of tracer uptake in order to
differentiate between osteomyelitis and soft tissue infection [8].
Fever of unknown origin:
Fever of unknown origin is defined as recurrent fever of 38.3? C or higher, lasting 3 weeks or
longer, and no diagnosis after appropriate inpatient or outpatient
evaluation [15,28]. Some authors have proposed a subclassification
including classic FUO in nonimmunecompromised patients, nosocomial
FUO, neutropenic FUO, and FUO associated with HIV infection [15].
There are numerous causes for FUO with infection (the most common
cause [28]) accounting for 13-43% of cases, and neoplasms for
15-25% [10,15,49]. Other causes include non-infectious
inflammatory processes such as collagen vascular disease,
vasculitis (up to 17% of cases of FUO [15]), granulomatous
diseases, and drug reaction [10]. In between 10-40% of patients,
the underlying disease may remain undiagnosed [15]. Tuberculosis
is the most common infection that causes FUP in developing
countries [28].
FDG accumulates at sites of infection and inflammation and FDG
PET imaging has been shown to be more sensitive and more specific
than Ga-67 scintigraphy for FUO evaluation [10,11,15].
Additionally- PET imaging can be performed in a much more rapid
manner [10]. The reported sensitivity is between 81-100%,
specificity between 81-90%, PPV of 81%, and an accuracy of 90% for
identifying the source of the FUO [10,15]. The negative predictive
value of a negative PET scan is also very (NPV up to 100%) high
thereby excluding a focal pathologic etiology for the patients
fever [18]. A meta-analysis comparing FDG PET/CT with gallium and
leukocyte scintigraphy found FDG had the best performance with a
summary sensitivity of 86%, specificity of 52%, and a diagnostic
yield of 58% [49]. Overall, FDG PET can provide helpful
information in identification of the source of the FUO in 25-69%
of cases [10,15,18,49].
Infective endocarditis:
Infective endocarditis is an uncommon, but serious complication
of valve replacement and has been reported in 0.3-6% of patients
with valve prostheses [38,47]. The sensitivity of transthoracic
echo for endocarditis ranges from 20-65% and for transesophageal
echo from 70-90% [44].
Patient preparation is essential for proper exam interpretation
[43]. At least 6 hours of fasting and 24 hours of a low
carbohydrate and fat-allowed diet are recommended [43]. FDG PET
imaging has been shown to have a sensitivity of 78-93%, a
specificity of 71-90%, a PPV of 68%, a NPV of 94%, and an accuracy
of 80% for prosthetic valve infection (compared to 64%, 100%,
100%, 81%, and 86%, respectively, for leukocyte scintigraphy)
[38,45]. Drawbacks of FDG imaging for endocarditis include poor
evaluation of the valve due to adjacent myocardial uptake and the
inability of FDG PET imaging to distinguish between infection and
inflammation [44].
False positive FDG exams can be seen in the first 1-2 months
following surgery (likely related to inflammation or foreign body
reactions) and this can affect the specificity and accuracy of the
exam [38,43]- although a negative exam performed within three
months of implantation has a high negative predictive value [47].
Other authors report that elevated uptake associated with
non-infected prosthetic valves can be seen as late as 8 years
following implantation [47]. Homogeneous uptake surrounding a
prosthetic valve may be considered a normal variant, especially if
it is mild in intensity [47]. Focal or intense uptake is more
likely related to infection [47]. Generally, intense FDG uptake
(prosthetic valve to background ratio > 4.4) is associated with
a high probability of infection [38]. Other authors suggest an SUV
max higher than 4 or a target to background ratio higher than 1.8
[44].
FDG PET is limited for the evaluation of native valve
endocarditis [44]. This is likely due to the small size of the
vegetation, lack of significant activated inflammatory cells, and
continuous movement of the valve [44].
In patient's with infectious endocarditis, up to 44% of patients
may have septic embolism and metastatic infection and whole body
acquisitions should be performed to properly identify these sites
[37,44]. Importantly, up to 50% of patients with septic embolism
do not have any localizing signs or symptoms and it is not
uncommon for there to be no clinical suspicion as well [37].
Reported sensitivity for metastatic infection is up to 100%,
specificity up to 80-87%, PPV up to 89-90%, and NPV up to 100%
[37]. One drawback of PET imaging in these patients is the lack of
ability to clearly identify endocarditis due to background cardiac
activity (sensitivity 39% in one study) [20,37].
In bacteremia/septic emboli:
S. aureus bacteremia is a severe infection associated with high
morbidity and a 30 day mortality of 20% [46]. Patients with
gram-positive bacteremia can develop infection at unsuspected
sites (metastatic infection) in 16-68% of cases [20]. These
metastatic foci of infection can be clinically silent (without
localizing signs or symptoms) in up to one third of patients
[20,46]. Risks factors for metastatic s. aureus infection include
community acquired bacteremia, signs of infection for more than 48
hours prior to initiation of appropriate antibiotic treatment,
fever for more than 72 hours following initiation of antibiotic
therapy, and positive blood cultures more than 48 hours following
initiation of appropriate antibiotic treatment [46].
Identification of these infectious foci is critical as prolonged
antibiotic treatment is usually required [20]. Insufficiently
eradicated infectious foci result in relapse infection in 12-16%
of patients once antibiotics are discontinued [20].
The use of 18F-FDG PET/CT can aid in the detection of
unsuspected sites of infection in these patients resulting in
lower relapse rates and mortality [20,37]. In one study of
patients with high risk bactermia, FDG PET identified metastatic
foci of infection in 74% of patients (more than two-thirds of whom
lacked signs or symptoms suggestive of metastatic complications)
[46]. Treatment modification in these patients resulted in a
significantly reduced 3 month mortality [46].
Patients on hemodialysis are also at higher risk for infection
[39]. PET/CT can aid in identification of the sites of infection
in up to 70% of patients [39]. The exam can also provide
prognostic information as FDG positive patients have been shown to
have a higher mortality (up to 26%) [39].
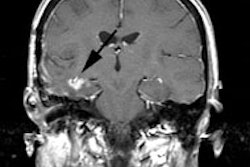
CNS infection:
FDG PET imaging can be used to aid in differentiating CNS toxoplasmosis infection from lymphoma in HIV patients [10]. CNS lymphoma typically demonstrates significantly greater FDG accummulation [10].
Spinal infection:
FDG PET imaging has proven to be very useful for the detection of disc space infection. Reported sensitivities of up to 100%, and specificities of up to 100% have been reported [2]. In general, increased FDG uptake is not seen in degenerative endplate abnormalities [2]. However, significant FDG uptake in degenerative spine disease occasionally occurs and foreign body reaction around uninfected spinal implants may also cause increased FDG uptake [42].
FDG-labeled leukocytes:
FDG-labeled leukocytes are also being studied for infection imaging. As with In-111 WBC's, the normal biodistribution of the tracer is to the reticuloendothelial system (bone marrow, liver, and spleen) [13]. Faint activity may be seen in FDG avid organs such as the brain and myocardium, but no significant renal or intestinal activity is seen [13]. Imaging can be performed 3-4 hours after injection, as opposed to the 24 hours required for In-111 WBC imaging [12,13]. Also- the tomographic nature of PET imaging enables more precise localization of sites of tracer accumulation [12]. The whole body and major organ dosimetry estimates from FDG-labeled WBC imaging are similar to those for In-111 WBC imaging [12]. The WBC labeling efficiency of FDG is lower than that of In-111 oxime (about 72% versus 90%) [12,15]. In vivo, about 25% of the activity is released from the leukocytes over 6 hours [15]. However, the overall sensitivity in one study higher than In-111 WBC's (87% versus 73%, but not statistically significant), while specificity, and accuracy were similar [12]. The accuracy was lowest for evaluation of osteomyelitis of the hands and feet [12]. False-positive exams can occur as a result of soft-tissue infection and physiologic accumulation of labeled leukocytes in granulating wounds [12]. Overall reported sensitivity is 86-87%, specificity 86%, PPV 92%, NPV 85%, and accuracy 86% [13].
PET Imaging in Inflammatory Conditions:
Sarcoid:
See also discussion in Chest section The ACE level can be used to monitor disease activity in
patients with sarcoid, however, it is elevated in only about 60%
of patients with chronic sarcoid and can be unrelated to disease
severity, progression, clinical course, and response to therapy
[29]. Sarcoid is a mutlisystem inflammatory disease and FDG
accumulation can be seen in active sarcoid lesions [14]. PET
imaging offers more rapid clinical evaluation compared to
traditional gallium imaging and is more accurate for the detection
of extrapulmonary sites of diease [14]. In patients with chronic
sarcoid, PET imaging can be used to detect sites of active
inflammation and the findings on the PET exam can affect therapy
decisions in up to 81% of patients [29].
FDG PET can also be used to evaluate for the presence of cardiac
sarcoid [30]. A limitation of FDG for the evaluation of cardiac
sarcoid is the unpredictable physiologic cardiac tracer uptake
even during fasting conditions that can interfere with detection
of active disease [30]. Various methods have been proposed to
decrease physiologic cardiac activity including prolonged fasting,
preadministration of unfractioned heparin, and use of a high-fat
low carbohydrate (HFLC) diet [30]. However, even when a HFLC diet
is used, diffuse homogeneous cardiac uptake can still be seen in
3-7% of patients and focal uptake in the papillary muscles can be
seen in 15-19% of patients [30].
For the diagnosis of cardiac sarcoid by FDG PET, in a metaanalysis the pooled sensitivity has been reported to be 89% (CI 79-96%) and specificity of 78% (CI 68-86%) [31]. The pooled prevalence of cardiac sarcoid in the studies was 50% which can obviously affect sensitivity and specificity [31]. In later stages that are characterized by fibrosis, FDG uptake may be low or not apparent [32].
The combined use of FDG PET and delayed enhanced cardiac MR may
provide optimal detection of cardiac sarcoid by allowing
differentiation of active granulomatous inflammation (MR-DE and
FDG positive) from fibrous lesions (MR-DE positive and FDG
negative) [30]. Correlation of the two exams can also help to
definitively characterize areas of FDG uptake and avoid false
positive exams associated with physiologic cardiac activity [30].
Vasculitis:
Patients with large vessel vasculitis typically have nonspecific constitutional symptoms of fever, malaise, weight loss, and an elevated ESR (small vessel vasculitis often results in hemorrhage and organ failure) [15,26]. Ultrasound can evaluate arteries in the proximal arm and axilla and can play a role in detection of giant cell arteritis (GCA) [21]. Sonographic findings include vessel wall edema (visible as hypoechoic circumferential wall thickening), vessel stenosis resulting in increased blood flow velocity and turbulence, and vascular occlusion [21]. On MRI, GCA demonstrates arterial wall thickening and abnormal gadolinium enhancement (sensitivity 81%, specificity 97%) [21].
PET imaging has been used to evaluate for large and medium-size
vessel vasculitis in patients with Takayasu's and giant cell
arteritis (generally for vessels that are larger than 4 mm in
diameter) [11,15]. PET imaging is not reliable for the diagnosis
of temporal artery inflammation [15]. 18F-FDG
localization within inflammed vessels is strogly correlated with
macrophage infiltration [22]. A characteristic feature of
vasculitis on PET is a circumferential region of increased
metabolic activity in the vessel wall [26].
FDG PET imaging is sensitive (77-92%) and highly specific
(89-100%) in the diagnosis of large-vessel vasculitis in untreated
patients with elevated inflammatory markers [15]. Reported NPV is
85%, and PPV is 100% [11]. PET imaging can also be used to monitor
disease activity and response to treatment [15]. One hour
post injection imaging appears adequate for the identification of
vascular inflammation and delayed imaging does not appear to
produce an additional advantage [19].
Atherosclerotic plaque:
FDG does not accumulate in normal vascular structures, but active
atherosclerotic plaque can be identified on FDG PET imaging [25].
When FDG uptake is seen, it is most commonly in large vessels
(over 1 cm in size) [26]. The presence of FDG uptake in the vessel
wall is indicative of active plaque containing macrophages-
focal intense tracer uptake has been proposed to be a marker
of lesions that are vulnerable to disruption (indicative of more
inflammatory cellular components within the plaque) [26]. In fact,
vascular FDG accumulation (such as in the carotid artiers and
aorta) has been demonstrated to predict an increased incidence of
future cardiovascular events [24,40].
18F-FDG uptake has been reported in coronary plaques,
but localization is difficult due to the small caliber of the
vessels, small size of the coronary plaques, coronary artery
motion, and normal background uptake of FDG by the myocardium
[27].
Uptake of FDG in abdominal aortic aneurysms has been reported to
be associated with active inflammation which can result in
weakening (due to release of the proteinaceous enzymes) of the
wall that can precede rupture [34].
REFERENCES:
(1) Radiology 2003; Schiesser M, et al. Detection of metallic implant-associated infections with FDG PET in patients with trauma: correlation with microbiology results. 226: 391-398
(2) AJR2002; Stumpe KDM, et al. FDG positron emission tomography for differentiation of degenerative and infectious endplate abnormalities in the lumbar spine detected on MR imaging. 179: 1151-1157
(3) Eur J Nucl Med Mol Imaging 2002; Zhuang H, et al. Persistent non-specific FDG uptake on PET imaging following hip arthroplasty. 29:1328-33
(4) Nucl Med Commun 2002; Chacko TK, et al. The importance of the location of fluorodeoxyglucose uptake in periprosthetic infection in painful hip prostheses. 23:851-5
(5) J Nucl Med 2003; Palestro CJ. Nuclear medicine, the painful prosthetic joint, and orthopedic infection. 44: 927-929
(6) Radiology 2004; Stumpe KD, et al. FDG PET for differentiation of infection and aseptic loosening in total hip replacements: comparison with conventional radiography and three-phase bone scintigraphy. 231: 333-341
(7) J Nucl Med 2004; Love C, et al. Diagnosing infection in the failed joint replacement: a comparison of coincidence detection 18F- FDG and 111In-labeled leukocyte/ 99mTc-sulfur colloid marrow imaging. 45: 1864-1871
(8) J Nucl Med 2005; Keidar Z, et al. The diabetic foot: initial experience with 18F- FDG PET/CT. 46: 444-449
(9) J Nucl Med 2005; Pellegrino D, et al. Inflammation and infection: imaging properties of 18F- FDG-labeled white blood cells versus 18F- FDG. 46: 1522-1530
(10) J Nucl Med 2005; Love C, et al. FDG PET of infection and inflammation. 25: 1357-1368
(11) Radiol Clin N Am 2005; Zhuang H, et al. Applications of fluorodeoxyglucose-PET imaging in the detection of infection and inflammation and other benign disorders. 43: 121-134
(12) Radiology 2006; Rini JN, et al. PET with FDG-labeled leukocytes versus scintigraphy with 111In-oxine-labeled leukocytes for detection of infection. 238: 978-987
(13) J Nucl Med 2006; Dumarey N, et al. Imaging infection with 18F- FDG-labeled leukocyte PET/CT: initial experience in 21 patients. 47: 625-632
(14) J Nucl Med 2006; Nishiyama Y, et al. Comparitive evaluation of 18F-FDG PET and 67Ga scintigraphy in patients with sarcoidosis. 47: 1571-1576
(15) J Nucl Med 2007; Meller J, et al. 18F-FDG PET and PET/CT in fever of unknown origin. 48: 35-45
(16) J Nucl Med 2007; Keidar Z, et al. Prosthetic vascular graft infection: the role of 18F-FDG PET/CT. 48: 1230-1236
(17) J Nucl Med 2008; High 18F-FDG uptake in synthetic aortic vascular grafts on PET/CT in symptomatic and asymptomatic patients. 49: 1601-1605
(18) J Nucl Med 2008; Keidar Z, et al. Fever of unknown origin: the role of 18F-FDG PET/CT. 49: 1980-1985
(19) J Nucl Med 2009; Menezes L, et al. Vascular inflammation imaging with 18F-FDG PET/CT: when to image? 50: 854-857
(20) J Nucl Med 2010; Vos FJ, et al. 18F-FDG PET/CT for detection of metastatic infection in gram-positive bacteremia. 51: 1234-1240
(21) J Nucl Med 2010; Gotthardt M, et al. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. 51: 193701949
(22) J Nucl Med 2011; Yoo HJ, et al. Vascular inflammation
stratified by C-reactive protein and low-density lipoprotein
cholesterol levels: analysis with 18F-FDG PET. 52:
10-17
(23) J Nucl Med 2011; Palestro CJ. 18F-FDG and
diabetic foot infections: the verdict is...52: 1009-1011
(24) Radiographics 2011; Stolzmann P, et
al. Complementary value of cardiac FDG PET and CT for the
characterization of atherosclerotic disease. 31: 1255-1269
(25) Radiographics 2011; James OG, et al.
Utility of FDG PET/CT in inflammatory cardiovascular disease.
31: 1271-1286
(26) Radiographics
2011;
James OG, et al. Utility of FDG PET/CT in inflammatory
cardiovascular disease. 31: 1271-1286
(27) J Nucl Med 2012; Cheng VY, et al.
Coronary arterial 18F-FDG uptake by fusion of
PET and coronary CT angiography at sites of percutaneous stenting
for acute myocardial infarction and stable coronary artery
disease. 53: 575-583
(28) AJR 2012; Nazar AH, et al. Spectrum of 18F-FDG
PET/CT findings in patients presenting with fever of unknown
origin. 199: 175-185
(29) J Nucl Med 2012; Sobic-Saranovic D, et al. The utility of 18F-FDG
PET/CT
for
diagnosis
and adjustment of therapy in patients with active chronic
sarcoidosis. 53: 1543-1549
(30) J Nucl Cardiol 2013; Soussan M, et al Clinical value of a
high-fat and low-carbohydrate diet before FDG-PET/CT for
evaluation of patients with suspected cardiac sarcoidosis. 20:
120-127
(31) J Nucl Med 2012; Youssef G, et al. The use of 18F FDG
PET in the diagnosis of cardiac sarcoidosis: a systematic review
and metaanalysis including the Ontario experience. 53: 241-248
(32) Radiographics
2011;
Maurer AH, et al. How to differentiate benign versus malignant
cardiac and paracardiac 18F
FDG uptake at oncologic PET/CT. 31: 1287-1305
(33) J Nucl Med 2013; Jamar F, et al.
EANM/SNMMI guideline for 18F
FDG use in inflammation and infection. 54: 647-658
(34) J Nucl Med 2013; Courtois A, et al. 18F
FDGuptake assessed by PET/CT in abdominal aortic aneurysms is
associated with cellular and molecular alterations prefacing
wall deterioration and rupture. 54: 1740-1747
(35) J Nucl Med 2014;
Schatka I, Bengel FM. Advanced imaging of cardiac sarcoid. 55:
99-106
(36) J Nucl Med
2014; Keidar Z, et al. 18F
FDG uptake in noninfected prosthetic vascular grafts:
incidence, patterns, and changes over time. 55: 392-395
(37)
J Nucl Med 214; Kouijzer IJE, et al. 18F FDG PET/CT for the detection of
septic embolism in patents with infective endocarditis. 55:
1045-1046
(38) J Nucl Med 2014; Rouzet F, et al.
Respective performance of 18F
FDG PET and radiolabeled leukocyte scintigraphy for the
diagnosis of prosthetic valve endocarditis. 55: 1980-1985
(39) J Nucl Med 2015; Tseng JR ,et al.
Clinical usefulness of 18F
FDG PET/CT for the detection of infections of unknown origin in
patients undergoing maintenance hemodialysis. 56: 681-687
(40) J Nucl Med 2015; Rudd JHF, et
al. Preciting aortic aneurysm expansion by PET. 56: 971-973
(41) Radiographics 2016; Dibble EH, et al. Role of PET/CT in
workup of fever without a source. 36: 1166-1177
(42) J Nucl Med 2016; Palestro CJ. Radionuclide imaging of
musculoskeletal infections: a review. 57: 1406-1412
(43) J Nucl Med 2016; Gomes A, et al. 18F
FDG PET/CT in the diagnostic workup of infective endocarditis
and related intracardiac prosthetic material: a clear message.
57: 1669-1671
(44) J Nucl Cardiol 2017; Pauliina S, et al.
18F FDG positron emission
tomography/computed tomography in infective endocarditis. 24:
195-206
(45) J Nucl Cardiol 2017; Hyafil F, et al.
Nuclear imaging for patients with a suspicion of infective
endocarditis: be part of the team! 24: 207-211
(46) J Nucl Med 2017; Berrevoets MAH, et al.
18F FDG PET/CT optimizes
treatment in Staphylococcus aureus bacteremia and is
associated with reduced mortality. 58: 1504-1510
(47) J Nucl Cardiol 2017; Scholtens AM, et
al. FDG PET/CT in prosthetic heart valve endocarditis: there is
no need to wait. 24: 1540-1541
(48) J Nucl Med 2017; Kagna O, et al. Does
antibiotic treatment affect the diagnostic accuracy of 18F FDG PET/CT studies in patients with
suspected infectious process. 58: 1827-1830
(49) AJR 2019; Sethi I, et al. Current status of molecular imaging of infection: a primer. 213: 300-308