Sarcoidosis:
View cases of sarcoid
Clinical:
The specific etiology of sarcoidosis
has not yet been identified, but it likely the result of an
interaction between environmental exposures and genetic
susceptibility initiating a cell-mediated immune response to as
yet unidentified antigens [8,65]. Some authors suggest that
propionibacteria and mycobacteria may be associated with the
development of sarcoidosis [69]. Familial clustering has been
reported in 4-17% of cases and there is a reported 80-fold
increased risk of developing sarcoid in monozygotic twins (which
supports genetic susceptibility) [65]. Thoracic sarcoidosis is a great mimic of other
diseases including lymphoma, tuberculosis, and many causes of
chronic pulmonary infiltrates. Sarcoid
begins as an alveolitis/ non-granulomatous pneumonitis
with a T-cell infiltrate. T-cells release factors which attract
macrophages which in turn form giant cells and non-caseating granulomas
within the interstitium. The end
result is interstitial fibrosis [4].
To confirm the diagnosis, granulomas of known causes and sarcoid-like reactions must be excluded [32]. Granulomatous lesions may result from TB, berylliosis, leprosy, hypersensitivity pneumonitis, and fungal disease [32]. Local sarcoid-like reactions may be seen in lymph nodes that drain a neoplasm or a site of chronic inflammation [32].
Patients generally present between the age of 20 to 40 years. Blacks (African-Americans) are affected more than whites (3 to 15:1) and females more than males (for the black population [9]). First-degree relatives of patients with sarcoidosis are at an increased risk for the disease [68]. Sarcoid is rarely seen in Afrian and South American blacks [9]. Swedes and Danes also seem to have a high prevalence of disease [32]. The disorder is rare in children and when it occurs, usually affects white males. Familial disease is seen much more frequently in blacks (17% of cases) [8]. Sarcoid seems to have an earlier onset and more aggressive clinical course in blacks [8]. Smokers are not an an increased risk for sarcoid, and smoking may have an inverse relationship to the disorder [8]. The development of sarcoid has been seen in association with interferon therapy [16]. Up to 7% of patients receiving interferon may develop sarcoid [16]. Sarcoid associated with interferon therapy manifests mainly as pulmonary (76%) or cutaneous disease with a benign, uncomplicated evolution [31].
Most patients are asymptomatic (25-50%). Among those with
symptoms, the most common are non productive cough, exertional
dyspnea, and wheezing [54]. Other symptoms include insidious onset
fatigue, low grade fever, and night sweats. Less commonly some
patients have chest pain (9-19% of cases [69]) or discomfort [54].
Pulmonary function tests typically demonstrate a restrictive ventilatory defect with decreased vital
capacity, FRC, and carbon monoxide diffusing capacity [32].
However, other authors indicate that PFT's can reveal either a
restrictive or obstructive pattern- interstitial disease results
in low lung complicance, while peribronchial lesions can obstruct small
airways [9]. Lab analysis in patients with sarcoid
reveals an elevated angiotensin
converting enzyme (ACE) level in 56-67% of patients with stage I,
72-87% with stage II, and 56-95% of patients with stage III
disease [4]. ACE levels may correlate with disease activity- ACE
is a product of macrophages and is an indicator of granuloma burden in the body [9]. Other
findings include anergy, peripheral lymphopenia (depressed cellular immunity),
and hypergammaglobulinemia. Hypercalcemia/ hypercalciuria
is found in 2-15% of patients and is
secondary increased intestinal absorption of calcium [19]. This
results from activated macrophages in sacroid
granulomas which are extra-renal
sources of 1,25-OH Vit. D. The Kveim-Siltzbach test is positive in 90% of
patients with active disease.
A diagnosis of sarcoid is based on clinical and radiologic
suspicion, tissue confirmation of noncaseating granulomas, and
exclusion of other diseases such as TB, lymphoma, or malignancy
[43]. The use of endosonographic nodal aspiration results in a
greater diagnostic yield compared to conventional bronchoscopic
biopsy (80-90% vs 53%, respectively) [43,54]. BAL fluid will
show a CD4+/CD8+ lymphocyte ratio greater than 3.5 (specificity
94%, sensitivity 53% for the diagnosis of sarcoid) and a
lymphocyte differential count of more than 15% (sensitivity as
high as 90%) [54].
It is important to remember that sarcoid is a systemic process which can involve other organ systems (extra pulmonary manifestations are seen in 30-50% of cases [54]). Pulmonary involvement is found in 90% of patients and mediastinal adenopathy is the most frequent finding [19]. Pulmonary parenchymal involvement is found in about 20% of patients [19]. The liver and spleen are the most commonly involved non-pulmonary viscera- with granulomata noted in 40-80% of patients in autopsy series [18,19]. Liver involvement is found in 24-94% of patients and liver lab abnormalities are seen in 2-60% of cases [18,21]. Symptomatic liver disease is uncommon (fewer than 5% of patients) [18]. Liver involvement is usually infiltrative, and focal liver lesions are uncommon (5% of patients) [21]. When focal involvement is present, the CT findings usually consist of multiple, small (0.5-1.5 cm) hypodense nodules [21]. On splenic biopsy, granulomas can be found in 24-59% of patients with sarcoid [18].
Other sites of involvement include bone (up to 15%), kidneys
(8-19% [18]), CNS (symptomatic disease in 5-9%- basal granulomatous meningitis; hypothalamus;
cranial neuropathy), skin/cutaneous (10% to 30%- erythema nodosum,
lupus pernio), occular (12-25%- uveitis), the myocardium (up to 25-50% of
patients, but only about 5% have clinical evidence of myocardial
involvement [19,28]), parotid glands
(6%) [19], and the testicles (5% or less of patients [18]). The
trachea is involved in 1-3% of cases- most commonly the subglottic region.
Head and neck involvement: Orbital structures are the most
commonly involved sites for head and neck sarcoid [55]. Uveitis is
the most common occular manifestation of sarcoid with anterior
uveitis seen in 65%, posterior uveitis in 30%, and a combination
in 10-15% of patients [55]. Patients complain of eye pain,
redness, decreased vision, light sensitivity, and dark floating
spots in the visual fields [55]. The optic nerve is the second
most common affected cranial nerve after the facial nerve
(bilateral facial nerve palsy may develop in up to one-third of
patients with neurosarcoid [65]) [55]. Optic nerve involvement can
present with decreased vision or vision loss, which can be rapid
and painful [55]. Involvement of the lacrimal gland occurs in 10%
of patients [55] and parotid gland involvement in 5% [65].
Involvement of the nervous system is seen clinically in 5-15% of
cases [66], but clinically silent CNS involvement can occur in up
to 25% of sarcoid patients [55]. Leptomeningeal involvement is the
most common manifestation of CNS sarcoid and can be seen in 40% of
affected patients [55]. Leptomeningeal sarcoid has a predilection
to involve the basilar meninges, most commonly the suprasellar and
frontal meninges [55]. Patients can present with HA, seizure, and
symptoms related to cranial nerve invovelment [55]. Dural
involvement is reported in up to 34% of patients and can appear as
focal or diffuse dural thickening [55]. Cranial nerve involvement
can be seen in up to 50% of patients with neurosarcoid [55]. The
most commonly affected nerves include the facial (VII), optic
(II), trigeminal (V), and oculomotor nerves [55]. Hypothalamic and
pituitary manifestations of sarcoid are rare and account for only
1% of lesions found within the sella [55].
Patients with cardiac involvement may demonstrate conduction abnormalities/arrhythmias, valvular dysfunction, and heart failure [33].
Syndromes associated with sarcoidosis:
Erythema nodosum (EN):
Most common non-specific skin finding in sarcoid [37]. EN can
occur in other nongranulomatous diseases as well [37]. The
disorder is a type of panniculitis (inflammation of the
subcutaneous fat) that manifests as multiple bilateral tender
erythematous nodules with diameters ranging from 1 to 10 cm most
commonly on the anterior shins [37].
Lofgren's syndrome refers to an acute febrile illness accompanied by bilateral hilar adenopathy and erythema nodosum in a patient with sarcoid [9,18]. They may also have uveitis or parotitis and arthralgias of large joints. These findings are associated with a favorable prognosis with complete resolution within 2 years of presentation. [4,54]
Heerfordt's syndrome: Consists of parotid gland enlargement/parotiditis, fever, uveitis, and cranial nerve (facial nerve) palsies [18,55]. The condition is usually self-limited and most commonly affects patients in the 2nd to 4th decades of life [19].
Lupus pernio refers to the
presence of violaceous (blue-purple)
raised skin lesions typically on the cheeks and nose or upper
torso in a patient with sarcoid [37].
Lupus pernio occurs more frequently among Puerto Ricans and
African Americans [37]. It is associated with a poor prognosis.
In HIV: A number of cases have been described of new onset sarcoid in HIV patients following initiation of antiretroviral therapy with rise in CD4 count [10,12]. This may be related to immune restoration [10,12]. The radiologic features are similar to sarcoid in non-HIV patients [12].
Treatment:
Many patients can be monitored without treatment because
spontaneous resolution or stability may occur (up to half of
patients with pulmonary sarcoid have spontaneous improvement
within the first 6 months) [54]. Steroids can relieve symptoms and
suppress inflammation. Indications for steroid therapy include
CNS, occular, cardiac and progressive
pulmonary involvement, as well as hypercalcemia. Between 50-90% of patients
will have a favorable response to corticosteroids, but relapse
after discontinuation of therapy is seen in 20-74% of cases [54]
and patients should be monitored for at least 3 years after
treatment [65]. Initial response to steroid treatment does
not preclude progression to pulmonary fibrosis. [9]. When steroids
cannot be tapered to 10 mg/d or less, methotrexate or other second
line agents may be considered [54[. For
the treatment of end-stage lung secondary to sarcoid,
lung transplant can be performed. Unfortunately, the disorder
recurs in the transplanted lung in up to 35% of cases [14].
Staging:
Staging is based upon the CXR findings (4): Stage 0- Normal CXR; Stage 1- Mediastinal/ Hilar adenopathy; Stage 2- Adenopathy plus parenchymal infiltrates; Stage 3- Lung infiltrates only (adenopathy has resolved); and Stage 4- Fibrosis/ Cystic changes.
At presentation about 5-10% of patients have stage 0 disease; 50%
(25-65%) have stage I; 25-30% have stage II; 10-15% stage III; and
5% have stage IV [9,32,65]. The
clinical course is variable, but nearly two-thirds of patients
will remain stable or experience a remission within a decade of
diagnosis [32]. Recurrence after a remission lasting more than 1
year is uncommon (fewer than 5% of patients) [32].
Prognosis is directly correlated with the patients staging. About 65-70% of patients (up to more than 90% [65]) with stage I disease will demonstrate spontaneous clearing of radiographic abnormalities and only about 7% will progress to stage II (4). About 30-50% of patients with stage II disease and 10-20% of patients with stage III disease have radiographs that return to normal. Overall about 20% of affected patients will progress to chronic lung disease/pulmonary fibrosis [32]. Non-whites tend to have extrathoracic manifestations and an overall poorer prognosis [9]. Other factors associated with a poor prognosis include disease onset after the age of 40 years, hypercalcemia, splenomegaly, osseous involvment, chronic uveitis, and lupus pernio [32]. Early-stage features that are associated with a good prognosis (spontaneous remission rate >85%) are fever, polyarthritis, and erythema nodosum [32]. There is a 5% mortality rate due directly to sarcoid [9].
Complications:
1- Pulmonary fibrosis:
About 10-20% of patients progress to stage 4 disease. The disorder proves fatal in only 4 to 10% of cases, usually the result of pulmonary fibrosis and cor pulmonale.
2- Mycetoma/Aspergilloma:
Fungal superinfection is a complication of stage 4 disease. Although the characteristic finding is that of a fungus ball within an apical cavity, pleural thickening adjacent to a known cystic space may be the earliest indication of Aspergillus superinfection- occuring 2-3 years prior to the appearance of an intracavitary fungus ball. The pleural thickening is often striking and may achieve a thickness of 2 cm or more [5]. The presence of air-fluid levels within sarcoid pseudocavities is another indication of superimposed fungal infection [32]. Because the walls of aspergillomas are supplied by branches of the bronchial arteries, they bleed easily and they can be associated with life threatening hemoptysis [32].
3- Pneumothorax:
Most cases occur in association with diffuse pulmonary parenchymal disease- particularly fibrocystic disease and are probably due to rupture of subpleural bleb. The incidence of pneumothorax is said to be higher in blacks than in caucasians- likely due to the greater likelihood for disease progression in blacks [5].
4- Cardiac involvement/arrhythmias:
At autopsy, cardiac involvement can be found in up to 25-27% of
patients with sarcoid (up to 75% of patients
in Japan [52]), however, clinical evidence of cardiac
involvement is found in only 5-7% of affected patients
[27,38,42,45]. Other authors indicate that only 40-50% of patients
with cardiac sarcoid at necropsy demonstrate any clinical evidence
of myocardial disease [42]. However, cardiac sarcoid accounts for
13-25% of disease related deaths [39]. The most characteristic
lesions of cardiac sarcoid are discrete, compact, non-necrotizing,
epitheliod granulomas along with patchy areas of fibrosis [67].
The diagnosis of cardiac sarcoid is difficult as blind
endomyocardial biopsy has less than 20-25% sensitivity in
detecting non-caseating granulomas because of the patchy
distribution of the disease [45,66]. In addition to a patchy
distribution, the lesions also have a predilection for the
subepicardial and mid-wall myocardium [67].
Myocardial sarcoid tends to involve the basal interventricular septum or left ventricular free wall (and can mimic HCM), with papillary muscle or RV involvement less common or only rarely seen [26,27,30,63,70]. Other authors indicate that the most frequently involved area is the ventricular septum (31.5%), followed by the inferior wall, anterior left ventricle, right ventricle, and lateral left ventricle [45,67]. Involvement of the interventricular septum accounts for the high rates of atrioventricular conduction abnormalities seen in affected patients [67]. Endocardial muscular biopsy can confirm the diagnosis, but has a low diagnostic yield (20-50%) due to the patchy nature of the disease and selective tissue sampling from only the right ventricle [34,42,48].
Cardiac involvement is a major adverse prognostic indicator in
sarcoidosis [34]. Up to 85% of sarcoid-related mortality results
from cardiac sarcoid [52]. Sarcoid involvement may result in
global or regional hypokinesis, with systolic dysfunction leading
to a reduced LVEF and an increased end-diastolic LV volume [35].
Inflammatory granulomas or post inflammatory scarring can disrupt
normal myocardial electrical activity and produce bundle branch
block, cardiac arrhythmias, heart block, CHF, and sudden death [22,27,34,45,48,67]. Involvment of the septum
favors conduction abnormalities - most frequent is third-degree AV
block which appears in 23-30% of cases [45]. Cardiac sarcoid
should be considered in patients younger than age 55 years who
present with unexplained second or third degree AV block [45].
Ventricular arrhythmias/tachycardia is the most frequent
arrthythmia occurring in up to 23-25% of cardiac sarcoid patients,
is the second most common clinical finding, and represents the
leading cause of sudden cardiac death in CS patients (CHF is the
second most frequent cause of cardiac death in CS patients)
[45,70]. The incidence of sudden cardiac death from dysrhythmias
in the context of cardiac sarcoid is between 12-65% [48].
Cardiac sarcoid should be considered in patients presenting with
second or third degree AV block of unknown etiology and in
patients under the age of 55 years, monomorphic ventricular
tachycardia in the absence of CAD [42]. Approximately 16-35% of
patients younger than 60 years who present with unexplained
complete AV block or ventricular tachycardia may have cardiac
sarcoid as the underlying cause [65].
Treatment: Corticosteroids are the principal treatment for
cardiac sarcoid [63]. The early initiation of steroid therapy can
be beneficial in recovery of AV nodal function, help to prevent
ventricular arthythmias, aid in preserving
LVEF, and improve long-term survival by inhibiting the
inflammatory response thereby limiting fibrotic formation in the
heart [22,52,63,71]. Some experts advocate 6-12 months of
therapy, but others recommend consideration of lifelong treatment
because of the risk for relapse or sudden death [63].
X-ray:
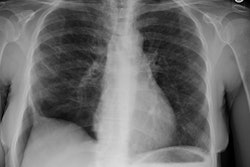
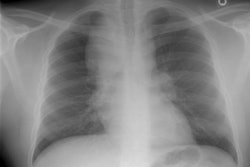
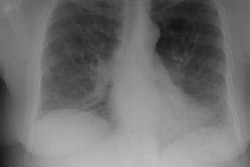
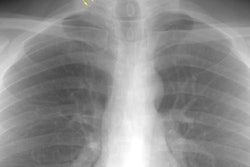
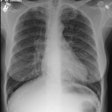
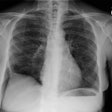
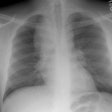
CXR: Plain film CXR abnormalities are found in over 90% of
patients with sarcoid at sometime
during the course of their disease (1). Bilateral hilar adenopathy
is found in 95% of cases, right paratracheal
adenopathy in 70%, and AP window adenopathy in 30-50%. Subcarinal nodes can also be involved in
up to 25% of patients. The presence of right paratracheal and
bilateral hilar adenopathy is referred to as the 1-2-3 pattern or
Garland triad [68]. The addition of AP window adenopathy is
referred to as the 1-2-3-4 pattern [68].
Lymph node calcifications are found in 3-20% (5). Calcification can be amorphous, popcorn-like, or uncommonly egg-shell in appearance (5%). Calcification of affected nodes is related to duration of disease. Calcification is seen in 3% of cases after 5 years, and in 20% of cases after 10 years [32]. RARE nodal findings include isolated mediastinal adenopathy (anterior, middle, or least commonly- posterior mediastinal adenopathy), or isolated unilateral hilar adenopathy (1-3%) (5). Older patients are more likely to have atypical patterns of adenopathy [9,32].
The interstitial lung disease can have a variety of appearances: a reticulo-nodular pattern is the most common and is typically bilateral and symmetric. An acinar or "alveolar" pattern with small indistinct nodular opacities can be seen. The densities can be discrete or coalesce producing areas of apparent parenchymal consolidation with air bronchograms [19]. The infiltrates can be peripheral and mimic pulmonary infiltrates associated with eosinophilia (1). Despite the appearance of airspace disease, the abnormality is still the result of interstitial disease. Multiple nodules have also been described, particularly in young black females and are usually very responsive to steroid therapy. Pleural effusions are RARE (0-5%), can be uni- or bilateral, are usually small to moderate in size, and are usually straw colored exudates with a lymphocyte predominance (5). A negative gallium scan is a reliable indicator of clinical inactivity.
Computed tomography: CT is more sensitive than CXR in the detection of adenopathy and parenchymal lung disease [9]. Mediastinal adenopathy is easily demonstrated.
HRCT is used to evaluate for parenchymal lung disease. Small 2-4 mm, well circumscribed pulmonary nodules are commonly seen (75-90% of cases) in a perilymphatic distribution [32]. The nodules are seen within the lung parenchyma (usually with a bilateral, symmetric, distribution predominantly in the upper and mid lung zones [32]), along the pleural surfaces, and along the fissures. There can be nodular thickening of the bronchial walls (when identified, this is associated with a very high diagnostic success rate for transbronchial biopsy (80-95%) [3]), along vessels, and along the fissures. These findings are related to the presence of non-caseating granulomas which are distributed within the bronchial mucosa, and along the lymphatics in the bronchovascular bundles, interlobular septa, and subpleural regions. Subpleural nodules (3-10mm in size) are found in 60-80% of cases. Large nodules greater than 1 cm in diameter occur as a result of coalescence of small nodules and are often surrounded by many tiny satellite nodules ("galaxy sign") [17,32]. Larger pulmonary nodules and masses can be seen in 15-25% of patients [32]. The masses may or may not contain air bronchograms [32]. Rarely, the nodules or masses may cavitate (<3% of patients) [32]. Other findings include ill-defined centrilobular nodules which may coalesce.
Nodular or irregular (occasionally smooth) thickening of the perivascular interstitium is also particularly common in sarcoid. The distribution of perivascular interstitial thickening may be patchy. Nodular or irregular thickening of the interlobular septae can be seen. Parenchymal bands can be seen in patients with sarcoid. Endobronchial granulomata in patients with sarcoid may rarely (1% of cases) result in significant airway narrowing [13].
Areas of ground glass attenuation can also be identified in about 40% of patients [32] and reflect the presence of microscopic interstitial granulomas or fibrosis, rather than alveolitis. Air trapping at the level of the secondary lobules has also been described and is felt to be related to narrowing of the small airways as a result of peribronchiolar granulomas [2,11]. The ground-glass opacities commonly have a finely stippled appearance due to the presence of tiny nodules [23].
Air trapping (due to small airways involvement by granulomas or fibrosis) will appear as areas of decreased attenuation on expiratory images- this produces a mosaic attenuation pattern [32]. Air trapping can be seen in up to 95% of patients [32].
Parenchymal opacities/airspace consolidation with air bronchograms may be seen in sacoid (alveolar sarcoid), but are uncommon (although other authors indicate that alveolar or airspace consolidation is seen in 10-20% of patients [32]). The consolidations are usually bilateral symmetric, and predominantly in the middle and upper lung zones [32]. The consolidation reflects the confluence of numerous micronodules that compress the surrounding alveoli or encroach on the alveolar spaces [32]. The extent of abnormalities on CT unfortunately correlate poorly with the patients level of functional impairment.
Pleural involvement in sarcoid is rare (1-4% of patients) [32].
In late stage disease, severe fibrosis results in architectural distortion, traction bronchiectasis, and fibrocavitary changes which is difficult to distinguish from other causes of end-stage lung disease.
Mesenteric and retroperitoneal adenopathy can be seen in approximately 30% of patients [40]. Liver involvement most commonly produces hepatomegaly, but focal nodules can be found in 5-19% of patients with liver disease [18,19]. When nodules are found, they are typically innumerable, diffusely distributed, and range in size from 1-2 mm to several centimeters [18]. The nodules appear hypodense on contrast enhanced CT imaging [18]. Hypodense splenic nodules can be found in about 6% to 35% of patients with sarcoid [18]. The splenic nodules are usually innumerable and diffusely distributed [18]. Intra-abdominal adenopathy can be found in up to 30% of patients [18].
Osseous involvement occurs in 1-13% of patients [65]. Skeletal lesions tends to involve the small bones of the hands and feet (in particular the distal and middle phalanges of the second and third digits [65]) and there is usually associated skin changes- soft tissue thickening referred to as "sausage dactylitis" [65]. Findings include a reticular trabecular pattern, a lace-like pattern of osteolysis, a mottled appearance, or cyst-like lesions [19,65]. The lesions are hot on bone scan and will also show tracer uptake on FDG PET imaging [20].
Cardiac involvement can appear as areas of myocardial thinning on
CT.
Neurosarcoid typically involves the leptomeninges (leptomeningeal
enhacement is most common in the suprasellar and frontal basal
meninges) and basal midline structrues, including the optic
chiasm, hypothalamus, and pituitary gland [40]. Cranial nerve
involvement can also be seen- most commonly the optic nerve, and
presents as an enlarged, enhancing nerve trunk [40].
MRI:
The appearance of cardiac sarcoid
depends on the stage of the disease [26]. Initial edema is
followed by granulomatous
infiltration that eventually leads to post-inflammatory scarring
[30]. The inflammatory phase is characterized by focal wall
thickening due to infiltration or edema, combined with wall motion
abnormalities on T1 cine images, increased signal on T2 images,
and early gadolinium enhancement [22,45]. DIR-FSE T2 images will
demonstrate edema as areas of foci of nodular high signal within
the myocardium preferentially affecting the mid wall and sparing the subendocardium [26,30,67].
Active granuloma may manifest as
focal nodular areas on cine images, appear bright on DIR-FSE T2
images, and will show delayed enhancement on post contrast imaging
(therefore LGE alone cannot determine phase of disease) [30,60].
Foci of delayed enhancement have been reported in 25% of cases
with biopsy proven disease and localize to the mid-myocardial
zones [34]. Involved areas can show enhancement on delayed T1
contrast enhanced images- the delayed enhancement may be mid-wall,
subepicardial, or transmural, rather than subendocardial [19,22,24,26,27,29] most
commonly in the basal and lateral aspects of the left ventricle
[29,57]. Other authors indicate that involvement is most commonly
transmural, and if non-transmural the involvement is often
subepicardial or midmyocardial [48]. Sarcoid has a
particular predilection for the basal or midventricular septum and
lateral LV wall and does not parallel the vascular territories
[34,35]. However, subendocardial hyperenhancement along the right
aspect of the interventricular septum was observed in 67% of
cardiac sarcoid patients in one study [35]. On cine MR, areas of
involvement may show segmental contraction abnormalities [22].
Delayed hyperenhancement and
myocardial thickening may resolve following steroid treatment
(although T2 hyperintensity usually
persists) [26]. MR has a sensitivity of 75-100% and specificity of
77-78% for the diagnosis of cardiac sarcoid [48,52,57]. Among
patients who are on already on steroid therapy, both CMR and FDG
PET may have reduced sensitivity, but CMR may perform better in
these patients [67].
In patients with cardiac sarcoid, delayed enhancement is
associated with adverse events, arrhythmias sustained ventricular
tachycardia, and cardiac death [45,48,57]. Sarcoid patients with
areas of delayed enhancement have a 11.5 to 20 times higher
likelihood of experiencing cardiac death compared to patients
without enhancement [57]. The presence of LGE should be considered
in clinical decision making for cardioverter-defibrillator
implantation [67].
Late in the disease, scarring will appear as focal areas of wall
thinning with associated hypokinesis
and delayed enhancement in a non-vascular territory affecting the
subepicardial layer [30].
Nuclear Medicine/PET in sarcoid:
FDG PET imaging will demonstrate tracer accumulation at sites of
active sarcoid involvement, can be
used to detect occult disease, and can also be used to monitor
treatment response [25,66].
PET imaging is more accurate and depicts extra-pulmonary
involvement better than gallium scintigraphy
[25]. Mediastinal lymph nodes are most commonly visualized (39% of
patients), followed by extrathoracic nodes (19%), and pulmonary
parenchyma (15%) [36]. FDG uptake is variable with SUVmax
measurements from 2.5 to 15.8 [36].
For evaluation of treatment response, in a small prospective
study, sarcoid patients with a metabolic response following
treatment with systemic corticosteroids had significantly fewer
relapses compared to patients without a metabolic response [66].
PET imaging of Cardiac Sarcoid:
The diagnosis of cardiac sarcoid is challenging - approximately
half of the affected patients are intially asymptomatic and
endomyocardial biopsy has a sensitivity of only 20-30% because of
the hetreogeneous myocardial involvement [52]. Gallium is specific
for cardiac sarcoid, but has a sensitivity of less than 40% [52].
FDG PET can also be used to evaluate for the presence of cardiac
sarcoid [41]. Unlike delayed enhancement on MRI which represents
cardiac damage, but can also represent scarring/fibrosis (chronic
disease), FDG uptake represents active inflammation [45,56]. A
limitation of FDG for the evaluation of cardiac sarcoid is the
unpredictable physiologic cardiac tracer uptake even during
fasting conditions (particularly in the inferolateral and basal
myocardium [45]) that can interfere with detection of active
disease [41]. Cardiac uptake tends to be higher in male patients,
patients under the age of 30 years, patients who fast less than
5-6 hours, patients with heart failure, and patients receiving
benzodiazepines [45,58].
Physiologic cardiac uptake is most likely to be minimized when
plasma free fatty acid levels are elevated (physiologic FDG uptake
is best inhibited when the FFA level is more than 760 uEq/L) [51].
A combination of fasting and diet modification can be used for
patient preparation in order to elevate FFA levels and suppress
physiologic FDG uptake by normal myocytes. The consensus
recommendation is that patients should consume at least two high
fat (>35 gm), low carbohydrate (<3 gm) protein permitted
meals the day before their exam and then fast for at least 4-18
hours [33,35,39, 41,42,45,47,51,58,63,64,70]. For patients that
cannot follow dietary recommendations, a fast of 18 hours or more
is recommended [63,64]. However, even when a HFLC diet is used,
diffuse homogeneous cardiac uptake can still be seen in 3-7% of
patients and focal uptake in the papillary muscles can be seen in
15-19% of patients [41]. Other authors suggest that despite
efforts to suppress myocardial activity, up to 30% of patients can
still have unsatisfying myocardial FDG uptake [70].
Injection of unfractionated heparin before FDG injection (in
conjunction with fasting and a low carbohydrate diet) can also be
performed to improve suppression of cardiac activity [42,53].
Unfractionated heparin increases plasma free fatty acid levels (an
up to 5 fold increase [53]) through activation of lipoprotein
lipase (thereby elevating plasma FFA levels and suppressing
glucose metabolism) and the effect occurs at a dose that is
significantly lower than that required for anticoagulation
[42,45,51]. A dose of 10 IU/kg (700-1000 IU) IV given in divided
doses 30 and 15 minutes prior scanning can be used (if there are
no contraindications) [42]. Other authors suggest using 50 IU/kg
of body weight 15 minutes before FDG adminstration [45,53].
Although the use of unfractionated heparin will acutely increase
FFA levels, some authors feel it does not have a significant role
in reducing physiologic LV FDG uptake compared to a prolonged fast
[51]. One potential concern with the use of heparin is the
uncommon, but potentially life threatening risk of heparin-induced
thrombocytopenia and no dose of heparin is too low to cause this
complication [59].
Intracellular calcium acts as a stimulus for cardiac glucose
uptake and the use of calcium channel blockers has also been
suggested as a means to decreased cardiac activity [47]. However,
this does not appear to be a useful addition to a standard
high-fat low carbohydrate meal followed by a 12 hour fast [47].
The consensus recommendation is that unfractionated heparin may
be considered, but it's actual impact on myocardial glucose
metabolism is uncertain [63].
Patients should be urged to avoid strenuous exercise for 12-24
hours prior to the exam [58]. Vigorous exercise and the resulting
catecholamine stimulation increase myocardial glucose uptake by
augmenting transporter recruitment and glucose utilization via
oxidation [58].
Consensus recommendations are that imaging should be performed
only at centers that have experience with cardiac sarcoid
protocols [62]. Results of the FDG exam should be interpreted in
conjunction with a rest myocardial perfusion exam- either 82Rb
or 13N-NH3 PET or resting perfusion SPECT using a 99mTc-agent
or 201Tl [63,70]. Findings in cardiac sarcoid include
focal or diffuse cardiac uptake (although other authors suggest
diffuse uptake is more likely non-specific [64]) [33]. A patchy
pattern of focal uptake is most suggestive of cardiac sarcoid
[45].
Early in the disease, the focal aeras of FDG uptake are not
associated with perfusion abnormalities, but typically there will
be a perfusion metabolism mismatch - area of FDG accumulation with
an associated resting perfusion defect (as also seen in areas of
hibernating myocardium and the pattern has been described in
myocarditis) [49,50,67]. On the other hand, resting perfusion
defects may be present in patients with cardiac sarcoid that has
no significant inflammatory component - therefore, the absence of
FDG activity should be interpreted as a sign of "no active
myocardial inflammation," but cannot exclude the presence of
cardiac sarcoid [67]. More diffuse uptake can occur if physiologic
myocardial suppression was insufficient [45].
| Perfusion |
Normal |
Normal |
Normal |
Abnormal |
Abnormal |
Abnormal |
| FDG |
Normal |
Diffuse (non-specific) |
Focal increase |
Focal increase same area |
Focal increase (different area) |
Normal |
| Interpretation |
Negative |
Non-specific |
Early disease |
Mismatch pattern (positive) |
Scar and inflammation |
Scar/No active inflammation |
From: Chest 1984; Glazer HS, et al. Peripheral pulmonary infiltrates in sarcoidosis. 86 (5): 741-744
(2) AJR 1997; High-resolution CT findings of pulmonary sarcoidosis. 168(6): 1557-1560 (No abstract available)
(3) J Thorac Imag 1995, 10: p.236-254 (p.239)
(4) Chest 1983; DeRemee RA. The roentgenographic staging of sarcoidosis: Historic and contemporary perspectives. 83 (1): 128-133
(5) AJR 1985; Rockoff SD, et al. Unusual manifestations of thoracic sarcoidosis. 144: 513-528 (Review article)
(6) ACR Syllabus #40:p.395-398
(7) Chest 1973; Sharma OP, et al. Nodular sarcoid: An unusual radiographic appearance. 64 (2): 189-192 (No abstract available)
(8) Semin Respir Infect 1998; Rybicki BA, et al. Epidemiology, demographics, and genetics of sarcoid. 13 (3): 163-173
(9) Radiographics 1995; Miller BH, et al. Thoracic sarcoidosis: Radiologic-pathologic correlation. 15: 421-437
(10) Society of Thoracic Radiology Annual Meeting 2000 Course Syllabus; Haramati LB. Emerging infections in AIDS. 117-119
(11) AJR 2000; Arakawa H, et al. Expiratory high-resolution CT: Diagnostic value in diffuse lung disease. 175: 1537-1543 (No abstract available)
(12) Radiology 2001; Haramati LB, et al. Newly diagnosed pulmonary sarcoidosis in HIV-infected patients. 218: 242-246
(13) AJR 2001; Marom EM, et al. Diffuse abnormalities of the trachea and main bronchi. 176: 713-717 (No abstract available)
(14) Radiology2001; Collins J, et al. Frequency and CT findings of recurrent disease after lung transplantation. 219: 503-509
(15) AJR 2001; Ravenel JG, et al. Sarcoidosis induced by interferon therapy. 177: 199-201 (No abstract available)
(16) AJR 2001; Wittram C, et al. Immune restoration syndrome manifested by pulmonary sarcoid. 177: 1427 (No abstract available)
(17) AJR 2002; Nakatsu M, et al. Large coalescent parenchymal nodules in pulmonary sarcoidosis: "sarcoid galaxy" sign. 178: 1389-1393
(18) AJR 2004; Warshauer DM, Lee JK. Imaging manifestations of abdominal sarcoidosis. 182: 15-28 (No abstract available)
(19) Radiographics 2004; Koyama T, et al. Radiologic manifestations of sarcoidosis in various organs. 24: 87-104
(20) AJR 2004; Aberg C, et al. FDG positron emission tomography of bone involvement in sarcoidosis. 182: 975-977 (No abstract available)
(21) AJR 2004; Jung G, et al. MRI of hepatic sarcoidosis: large confluent lesions mimicking malignancy. 183: 171-173
(22) AJR 2005; Vignaux O. Cardiac sarcoidosis: spectrum of MRI features. 184: 249-254
(23) AJR 2005; Miller WT, Shah RM. Isolated diffuse ground-glass opacity in thoracic CT: causes and clinical presentations. 184: 613-622
(24) AJR 2005; Tadamura E, et al. Effectiveness of delayed enhancement MRI for identification of cardiac sarcoidosis: comparison with radionuclide imaging. 185: 110-115
(25) J Nucl Med 2006; Nishiyama Y, et al. Comparitive evaluation of 18F-FDG PET and 67Ga scintigraphy in patients with sarcoidosis. 47: 1571-1576
(26) AJR 2007; Lim RP, et al. Non-ischemic causes of delayed myocardial hyperenhancement on MRI. 188: 1675-1681
(27) AJR 2007; Hansen MW, Merchant N. MRI of hypertrophic cardiomyopathy: Part 2, differential diagnosis, risk stratification, and post treatment MRI appearances. 189: 1344-1352
(28) J Nucl Cardiol 2008; O'Hanlon R, et al. Evaluation of non-ischemic cardiomyopathy using cardiovascular magnetic resonance. 15: 400-416
(29) AJR 2008; Ichinose A, et al. MRI of cardiac sarcoidosis: basal and subepicardial localization of myocardial lesions and their effect on left ventricular function. 191: 862-869
(30) Radiographics 2009; Sparrow PJ, et al. CT and MR imaging findings in patients with acquired heart disease at risk for sudden cardiac death. 29: 805-823
(31) Radiographics 2009; Kim YK, et al. Thoracic complications of liver cirrhosis: radiologic findings. 29: 825-837
(32) Radiographics 2010; Criado E, et al. Pulmonary sarcoidosis: typical and atypical manifestations at high-resolution CT with pathologic correlation. 30: 1567-1586
(33) J Nucl Cardiol
2011; Brancato SC, Arrighi JA. Fasting FDG PET compared to
MPI SPECT in cardiac sarcoid. 18:
371-374
(34) AJR 2011; Hoey ETD, et al. Cardiovascular MRI for assessment
of infectious and inflammatory conditions of the heart. 197:
103-112
(35) Radiographics 2011; James OG, et al.
Utility of FDG PET/CT in inflammatory cardiovascular disease.
31: 1271-1286
(36) Radiographics
2011;
Maurer AH, et al. How to differentiate benign versus malignant
cardiac and paracardiac 18F
FDG uptake at oncologic PET/CT. 31: 1287-1305
(37) Radiographics 2011; Kanne JP, et
al. Beyond skin deep: thoracic manifestations of systemic
disorders affecting the skin. 31: 1651-1668
(38) Radiology 2012; O'Donnell DH, et al. Cardiac MR imaging of
nonischemic cardiomyopathies: imaging protocols and spectra of
appearances. 262: 403-422
(39) J Nucl Med 2012; Youssef G, et al. The use of 18F FDG
PET in the diagnosis of cardiac sarcoidosis: a systematic review
and metaanalysis including the Ontario experience. 53: 241-248
(40) Radiographics 2013; Bhavsar AS,
et al. Abdominal manifestations of neurologic disorders. 33:
135-153
(41) J Nucl Cardiol 2013; Soussan M, et al Clinical value of a
high-fat and low-carbohydrate diet before FDG-PET/CT for
evaluation of patients with suspected cardiac sarcoidosis. 20:
120-127
(42) J Nucl Cardiol 2013; The role of of 18F-fluorodeoxyglucose positron emission tomography in guiding diagnosis and management in patients with known or suspected cardiac sarcoidosis. 20: 297-306
(43) JAMA 2013; von Bartheld MB, et al.
Endosonography vs conventional bronchoscopy for the diagnosis of
sarcoidosis. The GRANULOMA randomized clinical trial. 309:
2457-2464
(44) J Nucl Med 2014; Schatka I, Bengel FM.
Advanced imaging of cardiac srcoidosis. 55: 99-106
(45) J Nucl Med 2014; Schatka I, Bengel FM. Advanced
imaging of cardiac sarcoid. 55: 99-106
(46) J Nucl Cardiol 2014;
Osborne MT, et al. Reduction in 18F-fluorodeoxyglucose uptake on serial
cardiac positron emission tomography is associated with improved
left ventricular ejection fraction in patients with cardiac
sarcoidosis. 21: 166-174
(47) J Nucl Med 2014; Demeure F, et al. A
randomized trial on the optimization of 18F FDGmyocardial uptake suppression:
implications for vulnerable coronary plaque imaging. 55:
1629-1635
(48) Radiographics 2015; Jeudy J, et al.
Cardiac sarcoidosis: the challene of radiologic-pathologic
correlation. 35: 657-679
(49) J Nucl Cardiol 2015; Blankstein R, et
al. Proceedings of the ASNC cardiac PET summit meeting, May 12,
2014, Baltimore MD. 22. 4. Novel applications of cardiovascular
PET: 720-729
(50) J Nucl Cardiol 2015; Sperry BW, et al.
Infectious myocarditis on FDG-PET imaging mimicking sarcoid. 22:
840-841
(51) J Nucl Cardiol 2016; Manabe O,
et al. The effects of 18-h fast with low carbohydrate diet
preparation on suppressed physiological myocardial 18F-fluorodeoxyglucose (FDG) uptake and
possible minimal effects of unfractionated heparin use in
patients with suspected cardiac involvement sarcoidosis. 23:
244-252
(52) J Nucl Cardiol
2016; Bois JP, Chareonthaitawee P. Optimizing radionuclide
imaging in the assessment of cardiac sarcoidosis. 23: 253-255
(53) J Nucl Med 2016; Scholtens AM, et al.
Additional heparin preadministration improves cardiac glucose
metabolism suppression over low-carbohydrate diet alone in 18F-FDG PET imaging. 57: 568-573
(54) Mayo Clin Proc 2016; Carmona, EM, et al.
Pulmonary sarcoidosis: diagnosis and treatment. 91: 946-954
(55) AJR 2017; Chapman MN, et al. Sarcoidosis
in the head and neck: an illustrative review of clinical
presentations and imaging findings. 208: 66-75
(56) J Nucl Cardiol 2017; Lee PI, et al. The
role of serial FDG PET for assessing therapeutic response in
patients with cardiac sarcoid. 24: 19-28
(57) J Nucl Cardiol 2017; Manoushagian SJ, et
al. Multimodality imaging in the diagnosis and management of
cardiac sarcoidosis. 24: 29-33
(58) J Nucl Cardiol 2017; Osborne MT, et al.
Patient preparation for cardiac fluorine-18 fluorodeoxglucose
positron emission tomography imaging of inflammation. 24: 86-99
(59) J Nucl Cardiol 2017; Bhambhvani P.
Challenges of cardiac inflammation imaging with F-18 positron
emission tomography. 24: 100-102
(60) Radiographics 2017; Hashimura H,
et al. Radiologic-pathologic correlation of primary and secondary
cardiomyopathies: MR imaging and histopathologic findings in
hearts from autopsy and transplantation. 37: 719-736
(61) J Nucl Cardiol 2017; Ahmadian A, et al. The response of FDG
uptake to immunosuppressive treatment on FDG PET/CT imaging for
cardiac sarcoidosis. 24: 413-424
(62) J Nucl Med 2017; Ohira H, et al. Inter- and intraobserver
agreement of 18F-FDG PET/CT image
interpretation in patients referred for assessment of cardiac
sarcoid. 58: 1324-1329
(63) J Nucl Med 2017; Chareonthaitawee P, et
al. Joint SNMMI-ASNC expert consensus document on the role of 18F-FDG PET/CT in cardiac sarcoid
detection and therapy monitoring. 58: 1341-1353
(64) J Nucl Cardiol 2017; Chareonthaitawee P, et al. Joint SNMMI-ASNC expert
consensus document on the role of 18F-FDG
PET/CT in cardiac sarcoid detection and therapy monitoring. 24:
1741-1758
(65) Radiographics 2018; Ganeshan D, et al.
Sarcoidosis from head to toe: what the radiologist needs to
know. 38: 1180-1200
(66) Radiographics 2018; Akaike G, et al.
PET/CT in the diagnosis and workup of sarcoidosis: focus on
atypical manifestations. 38: 1536-1549
(67) J Nucl Med 2019; Ramirez R, et al.
Advanced imaging in cardiac sarcoid. 60: 892-898
(68) AJR 2020; Lee GM, et al. Sarcoidosis: a
diagnosis of exclusion. 214: 50-58
(69) Radiographics 2020; Naeem M, et
al. Noninfectious granulomatous diseases of the chest. 40:
1003-1019
(71) Radiographics 2020; Hotta M, et al. Radionuclide imaging of cardiac amyloid and sacoidosis: roles and characteristics of various tracers. 40: 2029-2041