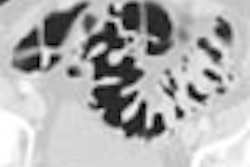
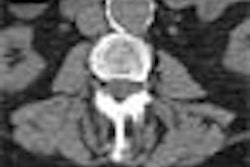
The Food and Drug Administration has cleared GE Medical Systems’ HiSpeed CT/e Dual, a dual-slice entry-level system that sells for between $528,500 to $728,500, depending on configuration.
CT/e Dual, which is being manufactured at the company’s GE Hangwei subsidiary in Beijing, will feature rotation times of 1 and 1.5 seconds, with slices as thin as 0.6 mm. The system brings multislice technology to a wider range of potential customers, according to the Waukesha, WI-based vendor.
By AuntMinnie.com staff writersJune 21, 2002
Related Reading
Medrad lands GE supply deal, April 30, 2002
CT: A market revitalized and redefined, April 5, 2002
Pediatric CT safety increased with new imaging techniques, March 12, 2002
GE uses ECR as CT launch pad, March 2, 2002
Copyright © 2002 AuntMinnie.com