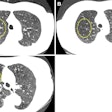
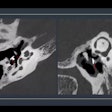
Schering and U.S. affiliate Berlex are voluntarily recalling all lots of its Ultravist Injection 370 mgI/mL x-ray contrast agent as part of a worldwide recall.
The firms are recalling the agents due to the potential that particulate matter in conjunction with crystallization may be present in the product. Potential for serious safety problems include thrombosis of blood vessels, thromboembolism, and injury or infarction of end organs such as heart, kidney, and brain, according to Schering of Berlin and Berlex of Wayne, NJ.
On July 20, Berlex announced the recall of a single lot of Ultravist.
The worldwide recall does not include other concentrations of Ultravist. In addition, production of Ultravist 370 mgI/mL in China and South Korea is not affected, and domestic supply in these two countries will continue, Schering said.
Proscope 370 mgI/mL in Japan and Clarograf 370 mgI/mL in Spain are affected by the recall, Schering said.
By AuntMinnie.com staff writers
July 31, 2006
Related Reading
Berlex issues Ultravist recall for U.S., July 21, 2006
Schering, Avid partner on Alzheimer's agents, July 17, 2006
Bayer reaches 88% stake in Schering, June 21, 2006
Schering divests CIS radiopharma unit, May 8, 2006
Berlex gets Magnevist renewal, August 4, 2005
Copyright © 2006 AuntMinnie.com