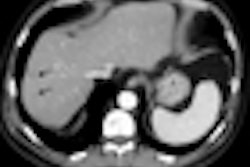
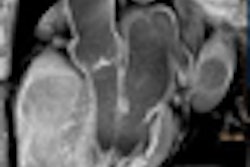
Computer-aided detection (CAD) firm iCAD of Nashua, NH, has submitted data to the U.S. Food and Drug Administration (FDA) for 510(k) clearance of its VeraLook CAD technology for CT colonography.
iCAD's VeraLook features advanced algorithms to detect and highlight polyps that may warrant a closer review by a radiologist.
Related Reading
iCAD gets Kansas City install, April 27, 2009
iCAD wins FDA nod for breast MRI software, April 16, 2009
iCAD launches CT colon CAD package, March 5, 2009
iCAD posts record 2008 results, February 26, 2009
iCAD debuts SecondLook with Planmed FFDM, December 31, 2008
Copyright © 2009 AuntMinnie.com