A noninvasive stool DNA methylation test unveiled on Thursday at the American Association for Cancer Research (AACR) meeting in Philadelphia showed high sensitivity and specificity not only for colorectal carcinomas, but also for advanced adenomas.
The authors, who analyzed data from more than 1,000 patients in their pilot study, believe that access to a sensitive and noninvasive colorectal cancer screening modality could sharply boost screening compliance in the U.S.
In an interview with AuntMinnie.com, lead researcher David Ahlquist, MD, professor of medicine and a consultant in gastroenterology at the Mayo Clinic in Rochester, MN, noted that only about half of those eligible for colorectal screening choose to be screened -- some objecting, undoubtedly, because current screening methods are burdensome.
"I think there's incentive and legitimate justification to look at improving screening approaches," he said. "An ideal strategy would be noninvasive and affordable, and this test comes a bit closer to the ideal; [with stool DNA], we have a chance to increase screening rates."
The stool DNA test is simple for patients, requires no dietary restrictions or bowel prep, and can be done from home with no lost work time. "Of course, it will be very important to link positive results to radiologic or endoscopic follow-up," Ahlquist said.
The technology was developed to incorporate direct capture from stool supermutant and quantitative allele-specific real-time target and signal amplification (QuARTS) assay technology. Reactions were used to assess genes for aberrant methylation of vimentin, NDRG4, BMP3, and TFPI2, the authors wrote in an accompanying poster presentation.
An earlier version of the test looked at only three premethylation markers, but the version used in the present study added K-ras mutation and hemoglobin markers as well, said Ahlquist, who has a financial interest in the technology as a consultant to Madison, WI-based Exact Sciences, and under an intellectual property sharing agreement between Mayo Clinic and the firm.
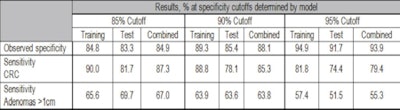
The research team examined 1,100 stored stool samples with a combination of methylation mutation and hemoglobin markers. The results showed sensitivities of 85.3% for detection of colorectal cancer (86.7% for curable cancers stage I though III) and 63.8% for adenomas larger than 1 cm at a specificity of 90%, Ahlquist said.
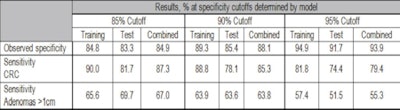 |
| Chart shows sensitivity for adenomas and colorectal cancers (CRCs), segregated by training and testing data as well as specificity or maximum false positives. |
Sensitivity results were reported at three specificity levels corresponding to three maximum false-positive rates, as shown in the chart above. At 90% specificity, or a 10% false-positive rate, "the test compares favorably to things like CT colonography [also known as CTC or virtual colonoscopy]," Ahlquist said. Thus, if the stool DNA test were given once every three years, it would deliver a cumulative 10-year false-positive rate of about 30%, compared to approximately 50% for current-generation stool testing kits.
"Programatically, we think the cumulative false-positive rate with this test would be lower than just about any other approach," Ahlquist said.
Study limitations include its retrospective design, which used frozen stool samples that had been kept for as long as seven years in some cases and potentially degraded the samples, Ahlquist said. But if anything, degraded DNA samples would have underestimated the sensitivity of the test, he added.
The retrospective study design was also to blame for a lack of quality control for the subsequent colonoscopies on which sensitivity and specificity were based. Future trials will incorporate prospective stool sample collection and colonoscopy quality control, he said.
The test will continue to be optimized in the run-up to an expected clinical trial for the U.S. Food and Drug Administration (FDA) that will include some 2,000 patients, Ahlquist said. In a best-case scenario, the test could be an available screening option within a couple of years. Current estimates peg the eventual cost of the new test at around $300, Ahlquist said.
The group is also exploring a "pan-cancer" gastrointestinal screening test that would incorporate markers sensitive to cancers above the colon, and it is reasonable to expect that a positive result for, say, stomach cancer could lead to endoscopic examination of the stomach, Ahlquist said.
For the colorectal cancer test, much work remains to be done, including standardization of the algorithms for what happens after a positive test -- for example, how to go about tracking down a positive result: in essence, what to do if VC and/or colonoscopy can't find a cancer, and how much additional diagnostic testing should be undertaken to find it. These questions are not easy, but they are eminently solvable, Ahlquist said.
The researchers concluded that DNA stool testing based on a combination of methylation mutation and hemoglobin markers achieved high detection rates for colorectal cancer and advanced adenomas with few false positives. Furthermore, detection rates were not influenced by the cancer stage or site of disease, they added.
By Eric Barnes
AuntMinnie.com staff writer
October 29, 2010
Related Reading
FOBT accuracy differs between men and women, September 7, 2010
CT-based Crohn's diagnosis takes aim at endoscopy, June 29, 2010
Colorectal cancer screening participation is highest with immunochemical test, December 31, 2009
Delayed return of fecal blood tests may miss colorectal cancers, August 11, 2009
Copyright © 2010 AuntMinnie.com