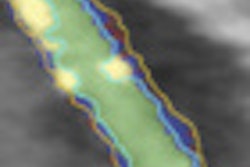
Computer-aided detection (CAD) developer Medicsight said it has received clearance from the U.S. Food and Drug Administration (FDA) for its ColonCAD medical image analysis software.
ColonCAD is designed to assist radiologists during their review of CT colonography images by automatically highlighting potential colorectal polyps. The FDA clearance was supported by a clinical trial involving 15 radiologist readers who each reviewed 112 CT colonography cases, both unassisted and assisted by ColonCAD, according to Medicsight.
The clearance allows Medicsight to implement its U.S. sales and marketing strategy, the company said.