Implementing iterative reconstruction (IR) on your CT scanner is more than just turning the thing on: You need a careful stepwise approach to get the most out of the radiation dose reduction technology, according to Dr. Jeffrey Mendel. In fact, a full review of your institution's CT protocols could be in order.
Proper implementation of iterative reconstruction is a big project because CT protocol review is an indispensable part of the process. You'll need to establish benchmark levels for radiation dose and adopt a healthy suspicion of your old protocols to get the most out of your IR software.
Perhaps most important, you'll need a strategy to get you from start to finish. While the process may seem to involve a lot of effort, you'll be handsomely rewarded with superior images, lower dose, and easier reading.
"There's no [relative value unit] directly for [IR] implementation, but on the other hand it will substantially improve the efficiency of your practice, so it's worth finding a champion in your practice who will drive it forward," said Mendel, who is an assistant professor of radiology at Tufts University School of Medicine. He spoke about iterative reconstruction in a talk at the International Symposium on Multidetector-Row CT in June.
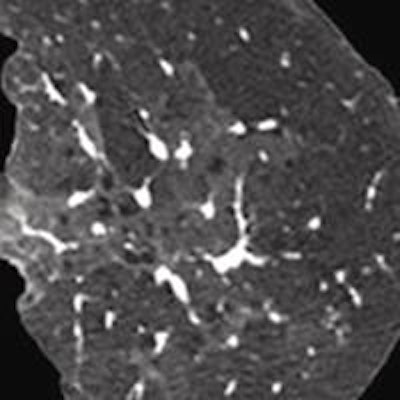
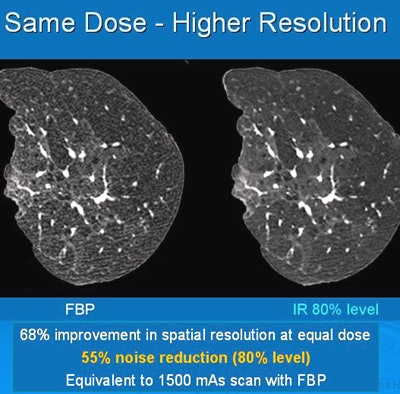 Iterative reconstruction provided 68% improvement in spatial resolution at an equal dose. All images courtesy of Dr. Jeffrey Mendel.
Iterative reconstruction provided 68% improvement in spatial resolution at an equal dose. All images courtesy of Dr. Jeffrey Mendel.In the past few decades, medical imaging's contribution to the average person's total annual exposure to radiation has risen from 15% to 48%, and CT is making up a larger percentage of that total. IR can turn the tide in the other direction, according to Mendel.
But maybe your department has been stopped in its tracks by the "paralysis of analysis," he said. After all, the past few years have seen an avalanche of papers covering everything from low kVp to dose reduction and iterative reconstruction. Lots of people fall into the trap of thinking the next best thing will make it worth their while to implement IR. And so they wait.
"It's sort of like Cher in 'Moonstruck,' " Mendel said. "Somebody has to hit you over the head and say, 'Snap out of it -- it's time to act!' So you can get beyond waiting for the next best thing and recognize that we're ready to implement now."
But implementing iterative reconstruction is more than buying it, turning it on at a modest level of dose reduction, dropping the mAs on all of your scans by 30%, and declaring victory, he said. Every department needs its own solution.
"If you haven't prepared adequately and if you aren't looking at individual cases for iterative reconstruction in your practice, then you aren't getting much advantage from it," Mendel said.
Effective utilization requires a stepwise approach:
- Identify and address your current quality assurance issues.
- Optimize your current dose reduction scheme, including tailoring exposure to patient body size, using automatic mA selection, minimizing the number of phases, and using low kVp to the extent possible.
- Select representative studies to use as a benchmark, and consider measuring image noise.
"The first thing you need to do is know how your doses compare to average doses," Mendel said.
An excellent information source is the American College of Radiology's (ACR) Dose Index Registry, and although it's best to actually join the registry, the dose data are available to nonmembers. The data will tell you the results of best practices around the U.S., rather than typical doses in the literature, which are not always ideal because most published studies do not account for differences in patient size, according to Mendel.
Measure some patients
Patients come in a wide variety of shapes and sizes, but neither weight nor body mass index (BMI) is a good way to measure them, he said. The most accurate way is the size-specific dose estimate (SSDE) developed by the American Association of Physicists in Medicine (AAPM). SSDE normalizes radiation dose based on body size using anteroposterior (AP) and lateral body dimensions.
 Dr. Jeffrey Mendel of Tufts University.
Dr. Jeffrey Mendel of Tufts University.Modern automatic exposure control (AEC) can help -- in fact, it works quite well in multiple applications, according to a recent article by Christner et al in Radiology. However, keep in mind that every vendor's AEC functions differently, and you need to know your scanner's functionality as well as its limitations, Mendel advised.
Even the newest, most automated systems still need some manual adjustment -- at a minimum, you should adjust for pediatric and bariatric patients, he said. If you have an older scanner, manual lookup using the AAPM tables works just fine.
"You can literally measure the AP diameter of the patient using the lookup table and get a pretty good measurement of what your patient's actual size is, and what you need to do with your protocol," Mendel said. To estimate a patient's "true" attenuation, you start with the AP scout image to measure width (skin to skin) at the level of the liver. Then use the AAPM tables to adjust exposure protocols.
SSDE takes CT dose index volume (CTDIvol) and converts it into a dose measurement, he explained. Other dose-reduction steps include modifying protocols to reduce mAs, cutting kVp, and limiting the number of imaging phases.
It's also important to remember that algorithms vary. Iterative reconstruction schemes all share some common general properties, but they "also take different approaches to things like artifact reduction," Mendel said. "So it's important to know your algorithm," as well as its strengths and limitations, to maximize utility.
The third step, selecting benchmark scans, involves reviewing your current CT protocols and choosing examples with typical good image quality for different patient types and sizes.
"Find a scan you really like to read in patients of varying sizes and various scan types and save those scans to use as a reference," he said. "Then calculate SSDE for those scans."
To calculate SSDE, you'll need dose reports and scout images, as well as a Web-based calculator. You can then further quantify your benchmarks by using the standard deviation from a region of interest (ROI) in addition to visual comparison to help measure and avoid unintentional "quality creep," Mendel said. Watching out for quality creep is important because no one will complain when image quality gets better than it needs to be due to overexposure.
Along with IR's well-known benefits -- reducing dose and decreasing streak and blooming artifacts -- it offers major advantages in increasing the apparent power (kW) of your CT scanner. This in turn enables the broader use of low-kVp techniques, improved image quality in bariatrics, and better lesion detection.
The apparent power increase can help you avoid situations in which peak-exposure demand exceeds system limits. How does this work? In tube power-intensive applications, the maximum power of a CT scanner may not be sufficient to provide the desired image quality. With iterative reconstruction, you can increase the apparent power without reducing mAs or kVp, and the more you dial up iterative reconstruction, the larger your apparent power gain.
"Your technologist may have seen what happens when you hit the tube limit," Mendel said. "To increase your resolution, you have to increase dose, so if you can't increase dose more, you're stuck."
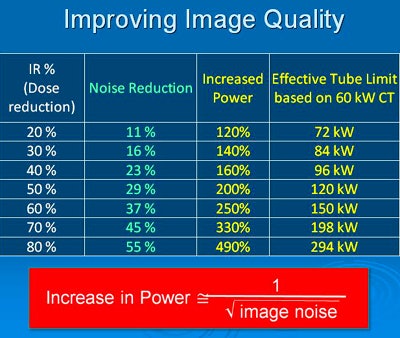 In the above example, the effective tube power increase using the iDose4 IR protocol (Philips Healthcare) provides image quality equivalent to that of a significantly higher-dose acquisition, without having to actually irradiate the patient with the higher dose. In such a scenario, tube power is increased enough to overcome the tube limits or skin dose concerns associated with higher-dose acquisitions.
In the above example, the effective tube power increase using the iDose4 IR protocol (Philips Healthcare) provides image quality equivalent to that of a significantly higher-dose acquisition, without having to actually irradiate the patient with the higher dose. In such a scenario, tube power is increased enough to overcome the tube limits or skin dose concerns associated with higher-dose acquisitions.Different departments, different approaches
How can iterative reconstruction be implemented in a department with multiple CT scanners from multiple vendors? Start with one scanner and your benchmark studies, Mendel said. Once you have honed a series of workable protocols, create IR benchmarks for use with your other scanners. Then quantify the new benchmarks just as you did with the original set of scans.
Perhaps your department is complicated by its division of labor -- for example, chest, abdominal, and pelvic studies might be read by different radiologists. In this case, implementing IR means getting agreement on protocols that affect more than one group.
"It's a little tricky, but you can heterogeneously apply IR across your department," Mendel said.
Many algorithms can be adjusted for IR level, he said. Knowing the age and experience level of your staff can offer clues for implementation. It could mean higher IR levels for younger radiologists and lower levels for more experienced radiologists.
"We did a study of residents, and we found that you could basically jack up IR to a level as high as possible and they were perfectly happy -- they hadn't spent 30 years looking at filtered back projection," Mendel said. "If you have a staff that's done that, you have to be a little more cautious and go a little more slowly in introducing IR."
Finally, rationing of some kind will almost certainly need to occur, because it's usually impossible to use your best scanner on every patient. "You need to figure out how to get your pregnant, bariatric, and high-dose study patients on those [IR] scanners, and you're going to have to have some limits in terms of scanner location and load balance," he said.
Once you're ready to implement IR, pick a level that's qualitatively acceptable and decide which patients need dose reduction versus image quality improvement. Next, try that IR level with 20 or 50 scans compared to your benchmarks and decide if you're happy, Mendel said. Then deploy that level throughout the department, or increase the IR levels a little and see how far you can go and still be comfortable.
"The status quo is someone else's solution to a previous problem," Mendel said, quoting retired Army Brig. Gen. Jack Hammond. "So unless you have an active system in your department that is pushing how you do your protocols, you have to assume the status quo was something that was old and somebody else worked on it. You have to work on it yourself. The bottom line is, keep pushing the envelope."




















