GE Healthcare has signed an agreement to provide miniature x-ray sources to colon capsule developer Check-Cap.
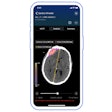
Check-Cap is developing a pill for colon cancer screening that includes a low-dose x-ray-based camera and wireless technologies that the patient swallows. The indigestible capsule will enable detection of colorectal polyps and cancer noninvasively and without the need for a laxative prep as it passes through the colon after being swallowed, Check-Cap said. It is aimed at increasing the willingness of individuals to undergo colon cancer screening.
Under the terms of the deal, GE Healthcare will develop and validate high-volume manufacturing for the production of x-ray sources, and it will assemble them into Check-Cap's capsule. The companies may also discuss collaboration on a high-volume manufacturing facility and distribution of the Check-Cap system.
Check-Cap is currently conducting a multicenter clinical feasibility study and expects to file a CE Mark submission for the Check-Cap system in the first half of 2017, the company said. The device is not currently cleared for clinical use in any geographic market.