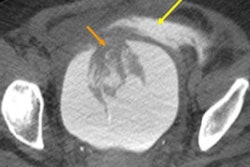
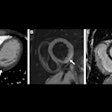
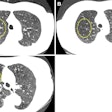
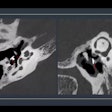
Computer-aided detection (CAD) firm Riverain Technologies has received U.S. Food and Drug Administration (FDA) clearance for its ClearRead CT nodule detection software.
ClearRead CT supports concurrent reading for all primary nodule types, including solid, subsolid, and ground-glass nodules. It's powered by acquisition normalization technology and processes CT scans from a range of manufacturers and acquisition protocols. No new hardware or customized tuning to specific devices or protocols is required.
ClearRead CT is comprised of two of Riverain's algorithms: ClearRead CT Vessel Suppress and ClearRead CT Detect.