On February 5, 2015, the U.S. Centers for Medicare and Medicaid Services (CMS) issued a national coverage determination (NCD) that found the evidence is sufficient to permit high-risk patients to receive annual low-dose CT (LDCT) scans for lung cancer screening without cost sharing.
High-risk patients were defined as individuals with no signs or symptoms of lung cancer who:
- Are between the ages of 55 and 77
- Smoked at least one pack of cigarettes every day for 30 years or more
- Are current smokers or ceased tobacco use within the last 15 years
As a result of the ruling, some 10 million Americans ages 55 to 77 at high risk for lung cancer are now eligible for lung cancer screening with guaranteed coverage and reimbursement by Medicare and many private payors.
Providing patients at high risk of developing lung cancer with annual LDCT scans has enabled radiologists to accurately diagnose and treat early-stage lung cancer and, subsequently, save more lives.
The economic imperative
The CMS ruling identified shared decision-making guidelines for referring physicians as well as minimum quality standards and requirements that imaging centers and radiologists must meet to receive reimbursement for providing or interpreting LDCT scans.
According to a CMS transmittal notice to Medicare contractors: "Before the first lung cancer LDCT screening, the beneficiary must receive a lung cancer screening counseling and shared decision-making visit, and if appropriate, receive the written order for his/her first lung cancer LDCT screen during such visit. Written orders for subsequent annual LDCT screens may be furnished during any appropriate visit with a physician or qualified nonphysician practitioner (physician assistant, nurse practitioner, or clinical nurse specialist) as described in the NCD."
The registry reporting mandate
Beginning in January 2016, Medicare began accepting claims for LDCT lung cancer screening retroactive to the February 5, 2015, date of the NCD. To be reimbursed by Medicare for lung cancer screening, LDCT providers must meet all eligibility criteria, including using an approved data registry for quality management.
The ACR Lung Cancer Screening Registry (LCSR) was approved by CMS to enable providers to meet quality reporting requirements to receive Medicare CT lung cancer screening payment.
"If a group is planning on seeing Medicare patients for lung cancer screening and wants to be reimbursed, they are required by CMS to collect and submit data to a CMS-approved registry," said ACR Health Policy Analyst Anita McGlothlin. "Currently, the ACR Lung Cancer Screening Registry is the only CMS-approved body."
Participation in the ACR Lung Cancer Screening Registry enables providers to meet quality reporting requirements and receive Medicare CT lung cancer screening payment. The LCSR collects data on patients, physicians, and outcomes of screening, using a registry structure based on the ACR's Lung-RADS reporting system.
Tips for registry participation
Here are six tips to guide you through the process of joining and participating in a lung cancer screening registry:
1. Register for the ACR National Radiology Data Registry (NRDR). Facilities that are already registered to participate in the NRDR can submit an LCSR participation agreement addendum and begin submitting their data. Note: Each facility must be individually registered in the NRDR to submit data.
2. Add all radiologists to your NRDR account. Before submitting your data, ensure that every radiologist conducting LDCT scans is registered.
3. Choose a method to submit data to the LCSR:
- Enter data manually using online forms.
- Upload a flat file -- bar (|) delimited -- configured according to the instructions in the LCSR User Guide (Section 4).
- Transmit data electronically using web-based services. To request IT specifications, please contact [email protected].
4. Submit your data at least quarterly. Capitalize on quarterly benchmarking reports to compare your quality and safety against a national standard.
5. Review the FAQs and other helpful guides:
- For participation in the LCSR: How to Get Started
- For data submission to the LCSR: LCSR User Guide
- For CMS FAQs: Coverage Information & FAQ
6. Ask the ACR for guidance. The ACR has experts available to answer questions and guide you through the process of participating in the LCSR. Email [email protected] or call 1-800-227-5463, ext. 3535.
The quality and safety opportunity
Beyond its role in ensuring Medicare reimbursement for LDCT scans, the ACR Lung Cancer Screening Registry helps radiology facilities benchmark and enhance the quality and safety of the lung screening care provided to their patients.
"Reimbursement isn't the only reason to participate in the ACR LCSR," said Mythreyi Chatfield, PhD, executive vice president of quality and safety at the ACR. "It's also about doing the right thing for patients. Facilities are submitting lung cancer screening data to the registry in order to benchmark their performance against a national standard for quality and safety."
As part of the NRDR, the ACR LCSR is a quality improvement tool that allows radiology facilities to monitor and demonstrate the quality of CT lung cancer screening in their practices through periodic feedback reports.
"The ACR registry will compile quality information that can help improve and refine lung cancer screening care at the national level," said Dr. Ella Kazerooni, chair of the ACR Lung Cancer Screening Committee and ACR Thoracic Imaging Panel.
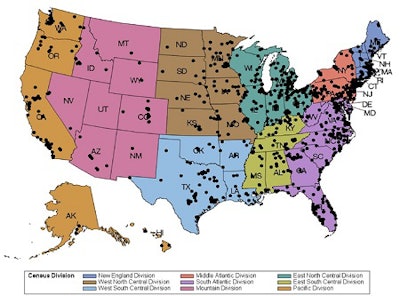
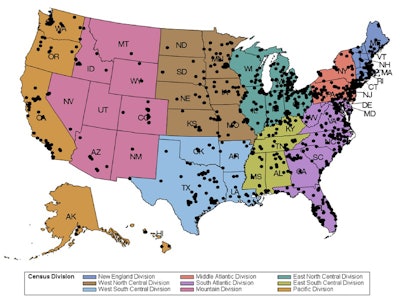
More than 1,500 radiology facilities are currently reporting quality and safety data using the ACR LCSR.
Tools and resources
The ACR has developed a suite of lung cancer screening resources to facilitate the diagnosis of lung cancer and help ensure quality care.
Visit the Lung Cancer Screening Resources page for information about providing safe and effective lung cancer screening with the latest research, toolkits, and key patient information.
Judy Burleson is senior director of quality management programs at the American College of Radiology.