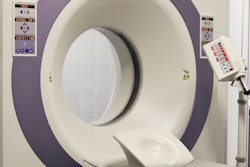
Faxitron Bioptics has received clearance from the U.S. Food and Drug Administration (FDA) for its VisionCT clinical CT scanner for intraoperative margin assessment of breast biopsies and lumpectomies.
The system consists of a motorized, 360° rotating platform that can acquire up to six high-resolution images per second, reconstructing the specimen in up to 1,024 slices at a standard slice thickness of 100 microns. It requires no manual specimen repositioning, and users can review orthogonal images while the specimen is being scanned and reconstructed, Faxitron said.