An artificial intelligence (AI) algorithm was able to predict patients' risk for future cardiovascular events by quantifying their coronary artery calcium (CAC) on routine chest CT exams, according to research published online January 29 in Nature Communications.
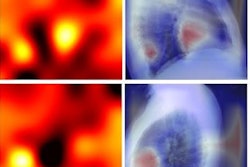
A team of researchers from Brigham and Women's Hospital (BWH) and Massachusetts General Hospital (MGH) developed a deep-learning model to automatically measure CAC on routine gated and nongated chest CT exams and then tested it on over 20,000 patients from four large clinical trials. They found that the algorithm's automated calcium score correlated highly with manual CAC quantification and was an independent predictor of cardiovascular events across multiple clinical scenarios.
"Implementation into clinical practice would address the unmet need of automating proven imaging biomarkers to guide management and improve population health," the authors wrote.
A key risk factor
Although CAC information should be available for almost every patient who receives chest CT, it typically isn't quantified due to time restrictions, according to senior author Hugo Aerts, PhD, of BWH's Artificial Intelligence in Medicine (AIM) Program. As a result, the researchers sought to develop an automated method for calculating this important risk factor for future cardiovascular events.
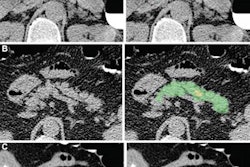
First author Roman Zeleznik of BWH's AIM Program and colleagues trained their end-to-end AI algorithm using data from 1,636 asymptomatic participants in the Framingham Heart Study (FHS). After localizing and segmenting the heart on chest CT exams, the deep-learning model then segments the CAC and calculates a calcium score. These scores are used to stratify patients into risk categories: very low (CAC = 0), low (CAC = 1-100), moderate (CAC = 101-300), and high (CAC > 300).
The researchers tested the algorithm on over 20,000 patients from four different study cohorts, including 663 from the FHS study, 14,959 heavy smokers receiving lung cancer screening in the National Lung Screening Trial, 4,021 patients with stable chest pain receiving cardiac CT (the Prospective Multicenter Imaging Study for Evaluation of Chest Pain, the PROMISE trial), and 441 patients with acute chest pain receiving cardiac CT (the Rule Out Myocardial Infarction Using Computer-Assisted Tomography, the ROMICAT trial).
After comparing the patient's AI-calculated risk category with patient outcomes, the researchers found that the automated score was a strong predictor -- independent of risk factors such as age, sex, diabetes, heart disease, hypertension, and stroke -- for future cardiovascular events in all patient cohorts included in the study. Patients deemed to be high risk based on the automated score had the highest hazard ratios -- up to 4.3 when compared with patients categorized as very-low risk, according to the researchers.
What's more, the algorithm's calcium score correlated highly (Spearman's correlation of 0.92) with human expert readers in a sample of 5,521 study participants. It also yielded a comparable area under the curve (AUC) to manual risk scores across three clinical trial cohorts, as well as demonstrated robust test-retest reliability in data from 252 individuals.
| AI performance for predicting cardiovascular events across test cohorts | ||
| Manually derived risk scores | AI-calculated risk score | |
| AUC | 0.75 | 0.74 |
| Test-retest reliability (intraclass correlation coefficient) | 0.997 | 0.993 |
The AUC difference did not reach statistical significance (p = 0.544).
The study shows the opportunity to use AI to glean additional value from chest CT exams, according to co-author Dr. Michael Lu of MGH's Cardiovascular Imaging Research Center (CIRC).
"The coronary artery calcium score can help patients and physicians make informed, personalized decisions about whether to take a statin," Lu said in a statement. "From a clinical perspective, our long-term goal is to implement this deep-learning system in electronic health records, to automatically identify the patients at high risk."
Co-author Dr. Udo Hoffmann of MGH's CIRC also noted that the algorithm's strong performance across the different study cohorts strengthens its generalizability to clinical settings.
The open-source algorithm can be downloaded for free from the group's website.