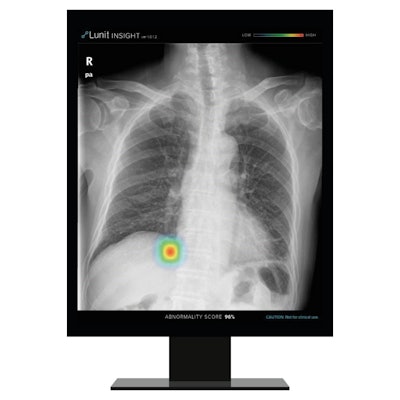
Artificial intelligence (AI) software developer Lunit has received clearance from the U.S. Food and Drug Administration (FDA) for its software to triage chest x-ray emergency cases.
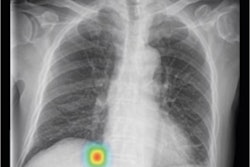
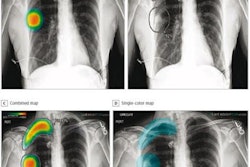
Lunit Insight CXR Triage prioritizes cases such as pleural effusion and pneumothorax by severity and transmits results immediately to physicians -- which reduces time-to-diagnosis, the company said. The application has been trained with more than 160,000 chest x-rays with CT images and has a sensitivity of 94% to 96% and a specificity of 95% to 99%, according to Lunit.