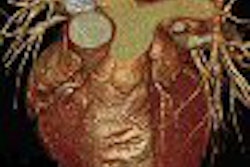
MRI contrast developer Epix Pharmaceuticals and marketing partner Schering have received clearance to market Epix's Vasovist blood-pool contrast agent in the European Union.
Vasovist, which will be marketed by Berlin-based Schering, has been approved for the visualization of abdominal or limb vessels in patients with known or suspected vascular disease using MR angiography, according to Epix of Cambridge, MA.
By AuntMinnie.com staff writers
October 5, 2005
Related Reading
Webb steps down at Epix, September 15, 2005
Epix sales fall, net loss grows, July 28, 2005
Epix makes management moves, July 6, 2005
Epix's Vasovist moves closer to market, July 1, 2005
Epix submits response to FDA, May 23, 2005
Copyright © 2005 AuntMinnie.com