The recent focus on rising CT radiation dose has some radiologists looking for alternative imaging tools, with digital radiography (DR) being one option. In part 2 of our two-part series on new DR technologies, we examine computer-aided detection (CAD) software and digital tomosynthesis.
Computer-aided detection
Researchers see CAD as a way to boost radiologist confidence in chest DR, potentially improving its sensitivity to a level closer to that of CT. Clinical trial experience with commercially available CAD software applications indicates that CAD makes DR much more sensitive to the presence of lung nodules, according to Dr. Edwin J. R. van Beek, Ph.D., chair of clinical radiology at the Queen's Medical Research Institute in Edinburgh, Scotland.
"It will catch most relevant nodules, but that may not be enough," he said. "Dual-energy digital subtraction may be necessary."
CAD systems are designed to serve as second readers by drawing the radiologist's attention to areas of an image warranting more analysis. In such instances, chest CAD backs up the radiologist's interpretation of chest DR or computed radiography (CR) images for the presence of pulmonary nodules.
Three products are cleared for sale in the U.S., while several more chest CAD products have received the CE Mark for marketing in Europe. IQQA-Chest CAD, developed by EDDA Technology of Princeton, NJ, and OnGuard 1.0 and 1.1 by Riverain Medical of Miamisburg, OH, are available in the U.S.
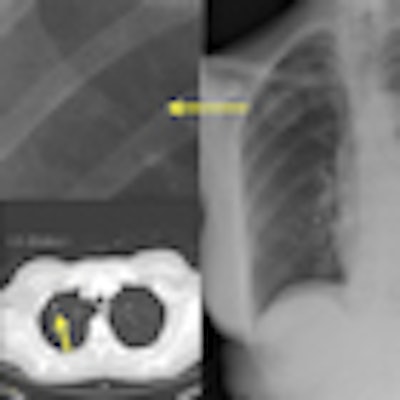
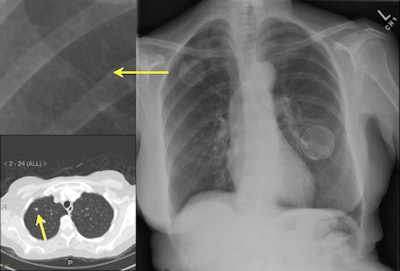 |
| IQQA-Chest software (right) identified two areas of potential interest. A magnified view (upper left) reveals a subtle mass; suspicious lung nodule later confirmed by CT (lower left) in renal cell cancer patient. The region of interest in the left lobe is a false positive. Image provided by EDDA Technology. |
Riverain is focusing new development efforts on OnGuard 5.0, a product that currently competes with IQQA-Chest CAD in world markets. Its application for U.S. Food and Drug Administration (FDA) premarket approval (PMA) is pending.
Because hypothesis-driven, multicenter trials are lacking, no solid conclusions can yet be made on how chest CAD affects reader accuracy. In fact, confidence in the entire field was shaken in 2007 with the publication of a retrospective study involving 31,000 screening mammograms read with CAD at 43 mammography facilities.
Dr. Joshua Fenton of the University of California, Davis found that that the software led to higher recall rates and a higher number of false positives that diminished overall reading accuracy (New England Journal of Medicine, 2007, Vol. 356:14, pp. 1399-1409).
Subsequent studies have contradicted these findings, but it was in the aftermath of Fenton's negative study that results from the first large-cohort study examining the efficacy of chest CAD were published. Van Beek reported encouraging results from a prospective trial that used six-month follow-up chest imaging to measure the accuracy of radiologists to detect lung cancer on DR with and without IQQA-Chest software (Academic Radiology, 2008; Vol. 15:5, pp. 571-575).
Follow-up imaging was performed on 214 patients or 67% of the individuals who were involved in the initial round of DR examinations. Initial imaging uncovered lung nodules in 35 patients (10%) without CAD and 51 patients (15%) when CAD backed up the radiologist's interpretation. Malignant lung disease was subsequently diagnosed in five of the additional 11 cases.
CAD was credited for raising exam sensitivity from 63.8% to 92.7%. Imaging specificity fell from 98.1% to 96.2% with CAD. The number of false-positive cases doubled from three to six.
Another retrospective study suggests that Riverain's OnGuard CAD could serve as an asset as well. In 2009, the University of Maryland's Dr. Charles S. White found that OnGuard 3.0, which preceded OnGuard 5.0, could potentially detect about half of missed cancers in clinical practice.
Missed lung cancers, ranging in size from 0.4 cm to 5.5 cm, appeared in 114 radiographs initially read without CAD. CAD performed up to 10 years after the initial reading identified 47% of the undetected lesions on a per-image basis and 52% on a per-patient basis, White reported (Radiology, 2009, Vol. 252:1, pp. 273-281).
Meanwhile, a Dutch study reported during scientific sessions at the 2009 RSNA conference found that radiologist resistance to using assistance presented by OnGuard 5.0 may be why the CAD software had no significant effect on the sensitivity or specificity of the readings.
Fifty-one patients with CT-detected, histologically proven lung cancers and 65 heavy smokers without nodules on CT were examined. On average, the four residents reading the studies ignored eight positive annotations from the CAD software, and radiologists ignored an average of 4.5 positive annotations. The investigators concluded that special training is needed to teach physicians to appreciate the importance of true positives and to rule out false positives with CAD.
Researchers are looking forward to definitive data from a five-year study of OnGuard 5.0 involving 9,000 subjects at the Cleveland Clinic. Dr. Moulay Meziane is the principle investigator of the trial, which is funded by Riverain. Plans for the study were announced in 2008, and patient enrollment was continuing in February 2010.
Digital tomosynthesis
Digital tomosynthesis is an old engineering idea that gained new life with the advent of flat-panel DR detectors in the 1990s. It is performed with a series of low-dose exposures acquired during a single sweep of the x-ray tube head around the patient. Imaging data are processed into slices showing anatomic structures at different depths and angles. The presentation creates a sense of depth and volume impossible with standard 2D radiographs.
Though tomosynthesis can be applied to body and chest imaging, most development work has revolved around mammography. Researchers have aimed at developing an instrument that brings volumetric perspective to breast cancer diagnosis without exposing the breast to huge amounts of ionizing radiation.
Early clinical experience with chest tomosynthesis indicates that patient dose is about 0.12 mSv, roughly three times more than with conventional DR, but about three times less than the 4 mSv received during chest CT, according a study from the University of Gothenburg in Sweden (Radiology, 2008, Vol. 249:3, pp. 1034-1041).
James Dobbins, Ph.D., an associate professor of radiology at Duke University in Durham, NC, believes tomosynthesis will occupy a clinical space between radiography and CT. Digital tomosynthesis provides exquisite resolution in the order of 0.2 to 0.4 mm in the 2D x- and y-planes.
In the z direction, it provides a tomographic perspective that DR alone does not provide, but the depth resolution is only about 5 mm for chest applications and about 1 mm in the breast. This means users will see fine detail in one plane and in only one slice, while some larger structures will show up to a lesser degree in adjacent slices.
"Tomosynthesis is not a replacement for CT. Rather, it is an improvement over conventional radiography by bringing in some 3D information," he said.
Dobbins characterized the current state of tomosynthesis as analogous with first- or second-generation MR scanners in the 1980s. FDA-cleared platforms from GE Healthcare (Chalfont St. Giles, U.K.) and Shimadzu Medical Systems North America (Torrance, CA) are commercially available, and additional products are working through the regulatory pipeline.
GE introduced VolumeRad, an option for the Definium 8000 DR platform, in 2006. It is also available on the Discovery XR650. Shimadzu launched the Sonialvision Safire in Japan in 2004. The company began selling an upgraded Safire II in the U.S. after FDA clearance in 2008.
Most clinical experience with tomosynthesis has revolved around breast imaging, where CT has a very limited role. Published data on its sensitivity and specificity are still limited, though a cohort study involving 513 women with abnormal screening mammography found that tomosynthesis and diagnostic mammography were both 92.9% sensitive to presence of malignant breast masses. The specificities of tomosynthesis and diagnostic mammography were 84.4% and 86.1%, respectively (European Radiology, January 2010, Vol. 20:1, pp. 16-24).
Medical literature covering chest tomosynthesis is limited. Theoretically, it is ideally suited for detecting pulmonary nodules, according to Dobbins, because it eliminates obstructions from overlying structures while producing images that look like a posteroanterior (PA) chest radiograph. It can be performed with few modifications to a conventional digital chest imaging room, so tomosynthesis could be acquired during the same imaging session as a conventional chest x-ray.
Early studies suggest that tomosynthesis improves upon conventional PA radiography. A study by Dobbins involving 21 subjects and 175 nodules (3-20 mm) demonstrated that pulmonary nodule detection improved from 22% with PA radiography to 70% with tomosynthesis over a wide range of nodule sizes (Medical Physics, 2008, Vol. 35:6, pp. 2554-2557). The high sensitivity rates documented in this study, and more recently published work from the University of Gothenburg in Sweden, were tempered by an increase in the incidence of false positives, Dobbins said.
More work is needed. The key translational issue for chest tomosynthesis, according to Dobbins, is determining the best clinical utilization strategy. He characterized it as a middle ground between chest radiography and CT. But for detecting pulmonary nodules, Dobbins envisions tomosynthesis as a vast improvement over conventional radiography rather than a replacement for CT. He outlined four possible utilization strategies:
- As a problem-solving tool for nodulelike opacities noted on radiography to avoid initial interrogation with CT
- As an adjunct to conventional radiography for high-risk patients, such as smokers older than 50
- For serial tracking of known nodules instead of using CT
- As an alternative to CT, if current large-scale clinical trials establish the value of routine CT lung cancer screening
As with dual-energy imaging and chest CAD, researchers working on digital tomosynthesis anticipate bright futures for technologies once clinical questions are worked out.
"The exact manner in which tomosynthesis will be implemented has still to be determined," Dobbins said, "but as more of these units are utilized clinically, an answer to that question will be forthcoming."
By James Brice
AuntMinnie.com contributing writer
March 25, 2010
Related Reading
Will new DR technologies offer alternative to CT? Part 1, March 24, 2010
Low-dose tomo beats chest DR for TB evaluations, March 19, 2010
Virtual dual-energy DR improves lung lesion detection, February 10, 2010
Rads prefer dual-energy x-ray over standard DR in lung, January 29, 2009
Adding CsI-based dual-energy imaging doesn't improve lung nodule detection, December 26, 2008
Copyright © 2010 AuntMinnie.com



















