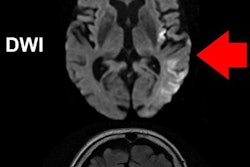
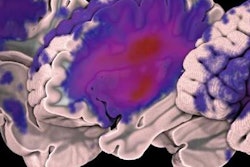
The U.S. Food and Drug Administration (FDA) has expanded the treatment window for use of medical device company Concentric Medical's Trevo image-guided clot retrieval device for stroke patients.
The device is used as an initial therapy for stroke to reduce paralysis, speech difficulties, and other stroke-related disabilities. Previously, it was cleared for use six hours after the onset of symptoms; the FDA has now expanded this to 24 hours.
Trevo is inserted through a catheter into the blood vessel to the site of the blood clot. A section at the end of the device expands up to 6 mm and grips the clot, allowing the physician to remove it by pulling it back through the blood vessel, the FDA said.