In an effort to make medical imaging more available to COVID-19 patients, the U.S. Food and Drug Administration (FDA) is relaxing its rules on modification of radiology equipment. Modifications will not need the agency's imprimatur before being used on patients during the crisis.
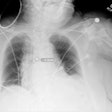
The COVID-19 outbreak has put stress on healthcare providers around the world as they struggle to meet the need to diagnose and treat a surge of patients. Radiology has faced unique complications, as it must balance the diagnostic capabilities of imaging technology with the need to protect staff from infection and disinfect equipment after use.
A number of radiology providers have resorted to creative strategies to provide services during the COVID-19 pandemic, such as placing mobile x-ray systems behind glass barriers that separate patients from staff. Other sites have reconfigured their facilities or changed staffing arrangements to reduce infection risk.
In an April 23 announcement, the FDA noted that it's become aware of imaging devices that have been modified into mobile or portable systems so that patients who might need imaging can undergo procedures without entering a large healthcare facility. To expedite availability of these modifications during the public health emergency created by the COVID-19 crisis, the agency said it did not intend to object to modifications of FDA-cleared or approved systems, even if it usually might require a 510(k) or premarket approval (PMA) application under normal circumstances.
The FDA provided a list of modifications to imaging equipment that it believed would not pose an undue risk to patients:
- Expansion of a clinical indication -- such as an extremity-only indication expanded to other body parts -- to acquire images where no alternatives exist in a facility
- Modifications that expand the mobility, portability, or relocation of an imaging system, such as the use of motors, batteries, or electrical components that would enable the conversion of a fixed imaging system to mobile use
- Modifications designed to protect the equipment operator or patient, such as a barrier
- Design changes that make it easier to clean, disinfect, or sterilize a system
- Changes to a device's components, such as the use of solid-state detectors, that do not seriously degrade image quality
The FDA also provided a list of device modifications that it would not look kindly on, due to their potential to create undue risk:
- Modifications that would reduce the amount of radiation shielding for patients or equipment operators
- Changes that would increase the radiation dose delivered to patients or result in insufficient image quality
- Modifications that would make it harder to clean, disinfect, or sterilize elements of devices that come into contact with patients
- Modifications that introduce new types of reconstruction algorithms
The FDA carved out a special section of the guidance for ultrasound systems, which have become useful in diagnosing patients with COVID-19. The agency said that because ultrasound scanners do not emit ionizing radiation, the FDA believes users can have "additional flexibility," with modifications allowed under the following circumstances:
- Modifications that would allow an ultrasound scanner to be used outside of the environment for which it is cleared, such as a general practitioner's office or a field hospital
- Changes that would enable the acquisition of images by healthcare practitioners who are not trained in sonography, under appropriate supervision
- The addition of lung scanning clinical applications, as long as labeling is included that is based on established practice guidelines, such as from the American Institute of Ultrasound in Medicine (AIUM)
- The availability of probes and machine settings that are suitable for lung scanning, such as probes at a frequency of 3 MHz and higher, and with linear, curvilinear, and phased-array technologies
- The use of a mechanical index (MI) < 1.4 as an indicator for potential biological effects on tissues containing gas bodies
The agency would frown on ultrasound modifications as follows:
- Changes that would result in an increase of the derated maximum acoustic output parameters
- Modifications that would exceed known safety limits in humans, such as a MI ≥ 1.4 for lung images
- Modifications that would allow lay users to acquire images without guidance of healthcare practitioners
Finally, the FDA said it would allow modifications to image analysis software. Allowable modifications include:
- Changes that add additional capability for lung segmentation and measurement
- Modifications that aid in the identification of nonspecific findings associated with COVID-19, such as ground-glass opacities on CT or Kerley B-lines on x-rays and ultrasound
- Modifications to image analysis software that would help evaluate and monitor patients with COVID-19 and their response to therapy based on quantitative metrics
Changes that would create patient risk include the following:
- Changes made to indicate that the device can be relied on to diagnose or triage COVID-19 on its own
- Modifications that claim a product can predict outcome and/or response to treatment
- Modifications designed to distinguish COVID-19 from other lung diseases
The FDA also recommended that manufacturers document changes to their products and that products include labeling that helps users understand the changes that have been made.
"In developing this policy, FDA's intent is to help expand the availability of imaging products, including mobile and portable diagnostic imaging systems, in the United States for the duration of the public health emergency while being flexible regarding the maintenance of devices," the agency wrote. "Wherever possible, healthcare facilities should use FDA-cleared or approved medical imaging systems and maintain this system following the manufacturer's or other recommended maintenance schedule."