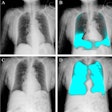
Artificial intelligence (AI) software developer Aidoc has received U.S. Food and Drug Administration (FDA) 510(k) clearance for AI-based software used for triage and notification of pneumothorax on x-ray exams. The software is Aidoc's first non-CT application.
The company said that current users of its software can add the pneumothorax software without any additional integration, infrastructure, or maintenance efforts. It runs on any type of x-ray system, including portable units, Aidoc said.
Aidoc has now received FDA clearance for eight AI software applications.