By Stephen T. Kee, MD, Stanford Medical School
Republished with permission from Applied Radiology, July 2000
The last two to three years have seen the development of new devices to assist with closure of arterial puncture sites following diagnostic and interventional endovascular procedures. The basic premise is that a medical device can be used to accelerate the time to hemostasis at the groin following removal of the in-dwelling sheath. Currently, most centers will pull the sheath and apply manual compression, a sandbag, or an external compression device to achieve hemostasis. Subsequently, the patient must lie flat for at least six hours and must be monitored continuously. This requires specialized nursing units and is a considerable strain on the resources of the hospital, and is also a source of inconvenience and discomfort for the patient.
A number of studies have also shown that simple manual compression is associated with approximately a 5% complication rate at the groin, most of which are relatively trivial, i.e., a small hematoma.1-3 However, a number of more serious complications can ensue, notably arterial pseudoaneurysms, arteriovenous fistulae, and infection. The overall cost to the hospital for the monitoring and management of complications can be substantial.
For this reason, a number of devices have been designed to increase the efficiency of arterial hemostasis, with a view to reducing complications and accelerating discharge of patients from the hospital. These devices are particularly useful in achieving hemostasis in patients who have undergone interventions requiring aggressive systemic anticoagulation and the use of antiplatelet agents. Often, achieving hemostasis in these patients can be extremely difficult. Arterial closure devices can be categorized into two different types: suture-mediated and nonsuture-mediated closure devices.
Suture-mediated closure devices
Perclose systems
The first suture-mediated closure device was manufactured by Perclose (Menlo Park, CA). Recently, this company was purchased by Abbott Laboratories (North Chicago, IL). The first generation Perclose device utilized a unique method of delivering needles and sutures into the vessel. The needles are deployed from the inside of the artery through the anterior arterial wall onto the skin. A knot is then tied and advanced down to the arterial wall using a knot pusher. Initial trials have shown Perclose to be extremely efficient, with over a 95% success rate in arterial closure.4,5 The Perclose device is available in either a Techstar or Prostar version. The Techstar device is a 6F device that has recently been upgraded to the Closer system.
Both the Techstar and Closer systems utilize two needles with one suture. The Closer device is advanced directly into the vessel over a standard 0.035-inch guidewire following removal of the in-dwelling sheath. The guidewire exits the device in a monorail fashion. As the device is advanced into the vessel, a unique "marking system" alerts the operator when the device is in the appropriate position, as blood is seen to exit from the hub of the device. A footplate is then deployed inside the vessel and the device withdrawn until it is felt to become taut against the inner surface of the anterior wall of the vessel. By pushing down on the plunger in the hub, needles are advanced through the anterior vessel wall catching sutures in the device footplate. These are then removed with the sutures attached, and are tied in a knot using a unique "clincher" knot-tying device. A pusher is then used to aid advancement of the knot to the anterior surface of the blood vessel (figure 1). Following successful closure of the vessel, patients can sit up immediately, are monitored for one hour, and discharged. The suture is manufactured from nylon and does not dissolve; it is left inside the patient permanently.
The Prostar system is available in 8F and 10F versions and uses four needles with two sutures. While Prostar devices use a similar system to the Closer, the needles are advanced from within the vessel to the skin surface. This system also has a larger hub configuration, which requires some degree of "tunneling" of the device down to the vessel surface. This device is somewhat more cumbersome than the Closer system and can be associated with a moderate amount of patient discomfort.
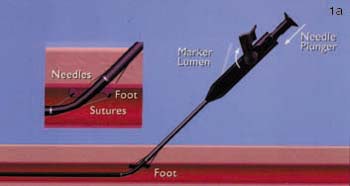
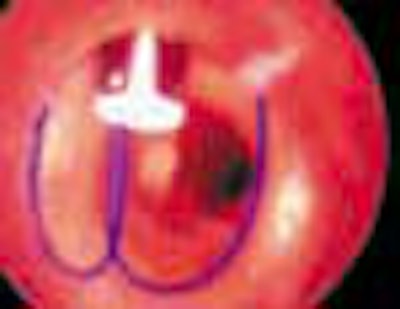
FIGURE 1. (A) Closer device (Perclose Inc., Menlo Park, CA) in situ with the footplate deployed in order to position the device accurately.

(B) Following removal of the needles, the sutures can be seen exiting the vessel wall through the hub of the device.
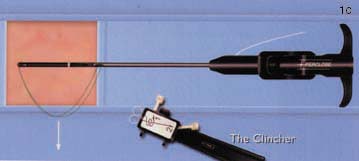
(C) The sutures are then removed from the device and inserted into the "clincher" knot-tying device.

(D) The knot is then advanced down onto the anterior wall of the vessel.
A number of operators have used the 6F Closer, and the 8F and 10F Prostar systems to close much larger arteriotomy sites. The basic technique involves deployment of the device during dilatation of the vessel. For example, for a 12F sheath, the arterial puncture is made in the usual fashion. Sequential dilatation is performed over a guidewire. After placement of the 8F dilator, the 8F Prostar device is placed and deployed. The untied sutures are left on the skin of the patient, the device is removed over a guidewire, and sequential dilatation continued until the 12F sheath is placed. Following the procedure, the knots are tied and the sheath removed while they are tightened. This "pre-close" technique, which has yet to be reported in the literature, is extremely useful and has been utilized to successfully close punctures of up to 24F in diameter.
Potential problems that can occur with the Perclose device include failure to tie a "slipping" knot. If the knot is tied inappropriately, it will not slide to the anterior vessel wall, resulting in device failure. When this occurs, mechanical pressure will be required to achieve hemostasis.
However, the development of the "clincher" device, which guarantees a properly tied knot, has significantly reduced this problem. The other potentially serious problem, which was a drawback of the first-generation Techstar device and continues to be a potential problem with the 8F and 10F Prostar device, is failure of the Nitinol needles to enter the hub of the device after exiting the anterior wall of the vessel. If this occurs, the physician can back the needles into the device and either retry or place a new device over the wire. Unfortunately, if the needles do not access the hub appropriately and the physician is either unaware or unable to correct the problem, the needles can become fixed in the soft tissues of the patient. This has required surgical retrieval of the needles. With adequate training and experience, this complication is extremely rare, and cannot occur when using the more modern 6F Closer device.
When used appropriately, the Perclose family of devices is extremely efficient and provides the operating physician with a great deal of confidence in the outcome of the closure. For this reason, the Perclose suture-mediated device has gained wide acceptance among interventional cardiologists, and its use in interventional radiology is growing rapidly.
Sutura
The SuperStitch device (Sutura, Fountain Valley, CA) is a new suture-mediated closure device that has yet to receive FDA clearance for use in percutaneous arterial closure. A variety of different-sized devices (6F to 18F) are used to close arteriotomies of up to 24F in diameter. The device is introduced into the vessel through the in-dwelling vascular sheath and a footplate is deployed. The device is withdrawn until the footplate is felt to encounter the anterior vessel wall. Needles are advanced from the device into the anterior wall of the vessel, picking up sutures in the footplate of the device. The needles are then withdrawn and sutures tied in the usual fashion (figure 2).
The basic mechanism of the device is quite similar to that utilized with the 6F Perclose system. The differences between the two devices are that the Sutura device does not enter the vessel over a guidewire; rather, it is placed through the in-dwelling vascular sheath. The SuperStitch device will also be available in a larger variety of sizes, up to 18F. The device has undergone trials in Europe, and recently received 510(k) clearance, limiting marketing of the device for general surgical or endoscopic procedures. A clinical trial to achieve approval for use of the device to close percutaneous punctures will begin this year. Initial results from trials in Europe are encouraging.
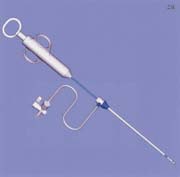
FIGURE 2. (A) Sutura SuperStitch arterial closure device shown inserted through a sheath.
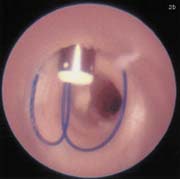
(B) Intra-arterial demonstration of the Sutura closure device with stitches running through the anterior vessel wall.
Non-suture-mediated devices
A number of devices (some FDA approved and others pending FDA clearance) are in use or in trials in the United States. All of these devices use some form of mechanical barrier or "plug" to close the vessel puncture site. Two devices approved by the FDA have gained widespread acceptance: AngioSeal and Vasoseal.
The AngioSeal device is currently marketed by St. Jude Medical (St. Paul, MN). An anchor plate placed within the vessel provides tension on a collagen plug, holding it in place on the outside of the vessel wall, thereby maintaining hemostasis. The system is delivered by removing the in-dwelling sheath and placing the 8F AngioSeal sheath within the vessel. The guidewire and dilator are then removed and the anchorplate delivered through the sheath into the vessel. The sheath is then removed, and tension maintained on the anchor plug until collagen has been placed outside the vessel wall. Pressure on the collagen is then maintained for 15 to 20 minutes using a spring system (figure 3). At this point, the spring is removed, and the device left in place to biodegrade. Complete absorption of the collagen plug and the anchorplate should take place in 30 days.
The device has shown good efficiency in early clinical trials, with a hemostasis rate of 95% to 98%.6,7 A number of complications have ensued from the use of this device, the most notorious being displacement of the in-dwelling anchor plug down the vessel, resulting in embolization at the popliteal trifurcation. While the anchorplug is made of reabsorpable material, it requires 30 days to be fully absorbed and embolization of this plug prior to this time will require surgical removal. This complication has occurred in only a small number of patients. The manufacturers received FDA clearance for a 6F rendition of this device in early 2000. While this version uses a somewhat modified anchorplate, the basic deployment of the device is the same. One significant downside of the device is the recommendation that the puncture site not be repunctured for at least 30 days. The same vessel can be accessed in a different location, however, there are concerns that puncturing a non-absorbed anchor device may be problematic.
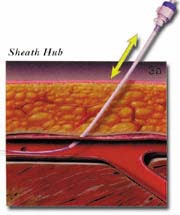
FIGURE 3. The AngioSeal sheath (St. Jude Medical, St. Paul, MN). (A) Following removal of the in-dwelling sheath, the sheath is advanced into position over the indwelling guidewire.
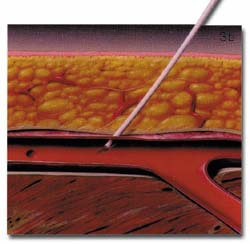
(B) The anchorplate is deployed within the vessel lumen.
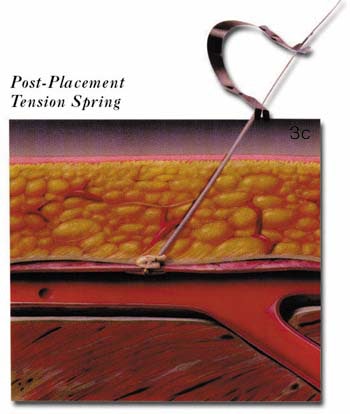
(C) The sheath is then removed and the collagen plug tamped in position on the anterior wall of the vessel using a tension spring.
The VasoSeal closure device (Datascope, Montvale, NJ) is somewhat similar to the AngioSeal device. It also uses a collagen plug pressed up against the anterior arterial wall at the puncture site; however, there is no prosthesis left within the vessel. The initial arterial sheath is removed over a guidewire and an 11F blunt tract dilator is used to dilate the skin to the anterior surface of the vessel wall. Through this tract, a plug of collagen is then pressed down against the arterial vessel wall to provide hemostasis (figure 4). Manual occlusive pressure must be provided up-stream from the puncture site while the collagen is deployed. This occlusive pressure is released gradually over a 30- to 60-second interval while non-occlusive manual pressure is initiated over the collagen plug. A number of complications have arisen from operators inadvertently delivering the 11F blunt tract dilator into the vessel. The Datascope device has also shown reasonable efficiency in initial clinical trials, approaching 95% hemostasis.8,9 Like the AngioSeal device, the vessel should not be re-entered for 30 to 60 days following delivery. Both the AngioSeal and VasoSeal devices use bovine collagen.
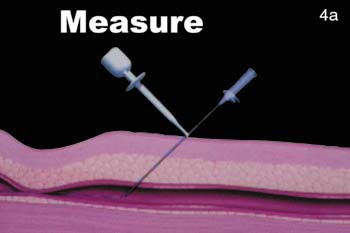
FIGURE 4. The VasoSeal device (Datascope, Montvale, NJ). (A) During the arterial puncture, the needle depth is measured.
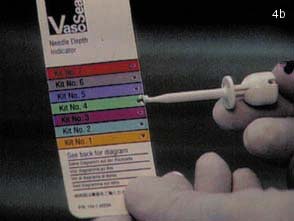
(B) Using the depth of the needle as a marker, the appropriate kit is opened.
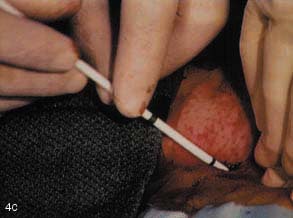
(C) The subcutaneous tract is then dilated using an 11F blunt dilator.
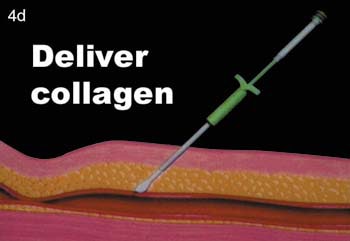
(D) The collagen is then delivered in position to the anterior wall of the vessel.
A number of pre-FDA clearance devices are undergoing trials in the United States, including the Duett device (Vascular Solutions, Minneapolis, MN). This device utilizes a 3F catheter advanced through an in-dwelling arterial sheath. The catheter has a balloon on the distal end, which is inflated. The sheath and catheter are then withdrawn from the vessel until the intra-arterial balloon becomes engaged in the anterior arterial orifice. A solution of thrombin and collagen is then delivered through the sheath into the periadventitial region of the artery. This solution promotes rapid development of an adherent clot at the anterior vessel surface, thereby promoting hemostasis. The one downside of this device is the potential for inadvertent delivery of thrombin and collagen into the vessel, which would result in significant intra-arterial thrombosis. A number of other manufacturers are developing non thrombogenic solutions to provide material plugging of the vessel wall, deployed in a similar fashion to the Duett device. These devices are all in pre-clinical stages and results have yet to be released.
Discussion
In 1998, approximately 9 million intra-arterial interventions were performed worldwide. Of these, less than 500,000 were closed using a mechanical closure device. The potential for an increase in this market share is the driving force behind further product development. The potential financial benefits to the company that secures a significant market share is so huge that these systems are likely to be here to stay.
Suture-mediated devices provide the clinician with the most reassuring feedback following successful closure, but they have a steep learning curve and potential complications and cost.
Non-suture-mediated closure devices may gain a foothold in smaller hospitals and in patients undergoing simple procedures, as these devices are less expensive. The cost to a hospital of a Perclose device is approximately $300 per unit, whereas an AngioSeal device costs approximately $150. Medicare and most HMOs do not pay for the procedure. However, hospitals seem willing to pick up the costs in return for reduced patient stay and complications. Any serious doubts in the profession that mechanical closure devices were simply a fad were removed when AngioSeal and Perclose were purchased by large corporations for $150 million and $650 million, respectively. This industry is here to stay, and it is beholden upon interventionalists to understand these devices, and to make their own decision as to which they feel is most beneficial to their patients.
Conclusion
In summary, a number of suture-mediated and non-suture-mediated closure devices are now approved and available to assist in achieving hemostasis following arterial interventions. When compared with the more traditional manual pressure, these systems are more efficient at achieving hemostasis, particularly in patients who have undergone interventions using anticoagulant or antiplatelet agents. Complications are low, and cost issues can be offset against more rapid discharge of patients. Additional devices are undergoing development and will be available in the next 12 to 24 months.
Dr. Kee is an Assistant Professor of Radiology at Stanford Medical School, Stanford, CA.
Let AuntMinnie.com know what you think about this story.
Return to Article Listing
References
1. Khoury M, Bantra S, Berg R, et al: Influence of arterial sites and interventional procedures on vascular complications after cardiac catheterization. Am J Surg 164:205-210, 1992.
2. Muller D, Shamir K, Ellis S, et al: Peripheral vascular complications after conventional and complex percutaneous coronary interventional procedures. Am J Cardiol 60:63-68, 1992.
3. Ricci M, Trevisani G, Pilcher DB, et al: Vascular complications of cardiac catheterization. Am J Surg 167:375-378, 1994.
4. Kussmal W, Buchbinder M, Whitlow P, et al: Rapid arterial hemostasis and decreased access site complications after cardiac catheterization and angioplasty: Results of a randomized trial of a novel hemostatic device. J Am Coll Cardiol 25:1685-1692, 1995.
5. Prostar: Instructions for use. Perclose Inc, Menlo Park, CA.
6. AngioSeal: Instructions for use. Sherwood Medical Company, St. Louis, MO.
7. Chevalier B, Lancelin B, Berrthaux X, et al: Hemostatic puncture closure device versus regular compression: A randomized study. J Am Coll Cardiol Special Issue 92A:16(Feb), 1995. Abstract #918.
8. VasoSeal: Instructions for use. Datascope Corporation, Montvale, NJ.
9. Sanborn TA, Gibbs HH, Brinker JA, et al: A multicenter randomized trial comparing a percutaneous collagen hemostasis device with conventional manual compression after diagnostic angiography and angioplasty. J Am Coll Cardiol 22:1273-1279, 1993.















