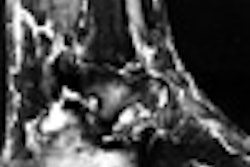
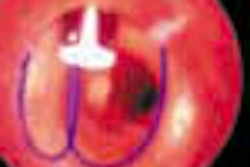
Stent manufacturer Guidant has launched a clinical trial to test its Neurolink stenting system in 30 patients with cerebrovascular disease, the company said. SSYLVIA (Stenting in SYmptomatic Atherosclerotric Lesions of Vertebral and Intracranial Arteries) is a prospective trial that will enroll approximately 30 patients with cerebral atherosclerosis. Stenting with Neurolink will be performed with the goal of preventing ischemic stroke, according to Indianapolis-based Guidant.
The Neurolink system is designed to treat atherosclerosis of the intracranial arteries and extracranial vertebral arteries. The first two patients were successfully treated on November 9, Guidant said.
SSYLVIA trial co-investigator Dr. Helmi Lutsep of Oregon Health Sciences University said endovascular stenting is a potentially important advance in the treatment of patients with cerebrovascular atherosclerosis who are at high risk for stroke, and whose treatment options are currently limited to drug therapy.
According to Dr. Stanley Barnswell, who performed the first Neurolink procedure last week, the system offers the flexibility needed to access cerebrovascular lesions. A lack of flexibility has limited development of effective treatments until now, he said.
By AuntMinnie.com staff writers
November 13, 2000
Guidant stent for large arteries cleared for market, September 11, 2000
Guidant launches peripheral stent trial, August 30, 2000
Boston Scientific, Guidant bury hatchet, May 17, 2000
Copyright © 2000 AuntMinnie.com