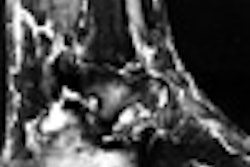
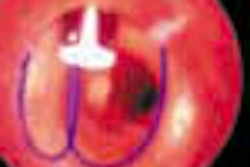
Interventional device developer Guidant has begun a clinical trial to evaluate the efficacy of carotid artery stenting as a means of preventing stroke. The study will compare the procedure to the standard treatment method of carotid endarterectomy.
The multicenter research is being funded by the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health (NIH) and will enroll 2,500 patients at 60 centers in the U.S.
The Indianapolis-based company’s ACCULINK has been selected as the exclusive stent for the trial. The product, available in both tapered and straight configurations, is designed to open carotid arteries that have been occluded due to atherosclerosis.
By AuntMinnie.com staff writersDecember 22, 2000
Related Reading
Guidant seeks FDA approval for intravascular radiotherapy system, December 22, 2000
Guidant begins cerebrovascular stenting trial, November 13, 2000
Guidant stent for large arteries cleared for market, September 11, 2000
Guidant launches peripheral stent trial, August 30, 2000
Boston Scientific, Guidant bury hatchet, May 17, 2000
Copyright © 2000 AuntMinnie.com