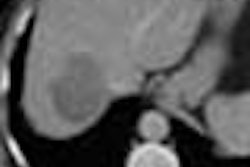
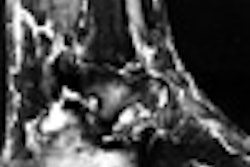
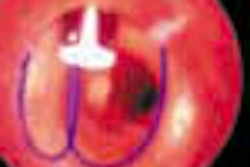
Interventional firm C.R. Bard has received Food and Drug Administration clearance to market its Memotherm endoscopic biliary stent for the treatment of malignant biliary obstructions.
The stent is preloaded on a flexible delivery catheter that allows it to maneuver easily through narrow and winding anatomy, according to the Murray Hill, NJ-based vendor. Upon deployment, the nitinol stent automatically expands in response to body temperature, and applies constant radial pressure to open the passageways of the duct and restore the flow of bile, according to Bard.
By AuntMinnie.com staff writersApril 17, 2001
Related Reading
Bard to test ePTFE-covered stent, March 8, 2001
Bard gets clearance for Luminexx, March 6, 2001
Copyright © 2001 AuntMinnie.com