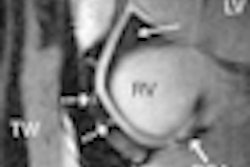
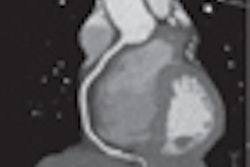
Coronary imaging developer InfraReDx of Burlington, MA, is touting the results of a case report that describes the association of stent thrombosis with lipid-core plaque as detected by the company's LipiScan coronary imaging system.
A case report in the December 7 issue of Circulation describes the occurrence of thrombosis in a stent that ends in a lipid-core plaque, which contains thrombogenic substances. This factor adds to the accumulating evidence that many complications of stenting, such as peristenting infarction, stent thrombosis, and restenosis, are due to the presence of a lipid-core plaque at the stented site, rather than features of the stent or stenting technique.
LipiScan is the first system cleared by the U.S. Food and Drug Administration (FDA) to provide a chemogram, or map of lipid-core plaque, within an imaged vessel through near-infrared (NIR) spectroscopy.
The case represents the first demonstration of acute stent thrombosis associated with disrupted lipid-core plaque as detected by NIR imaging with the LipiScan system, the study's authors noted.
Related Reading
InfraReDx secures $21M in financing, October 12, 2010
InfraReDx gets FDA nod for IVUS system, September 1, 2010
InfraReDx announces first clinical use of LipiScan, May 16, 2008
FDA clears InfraReDx's LipiScan, April 30 2008
InfraReDx receives FDA clearance, October 19, 2006
Copyright © 2010 AuntMinnie.com