With so many well-developed imaging tools available, it might be tempting to underestimate the hard work that preceded the development of FDG-PET. But the researchers who worked to develop it can testify to the many years of research and numerous participants required to create this major new diagnostic modality.
In this article, Dr. Abass Alavi and Dr. Martin Reivich of the University of Pennsylvania in Philadelphia describe the initiatives taken by themselves and others that led to FDG-PET and advanced its use. They also ponder the future potential for PET imaging, and how it may be compromised by the many challenges of modern healthcare.
FDG-PET: A theoretical basis
The concept of emission and transmission tomography was introduced by University of Pennsylvania scholars David Kuhl and Roy Edwards in the late 1950s, which later led to the design and construction of several tomographic instruments at Penn. These machines were able to successfully map the regional distribution of radionuclides, such as technetium-99m, as tomographic images.
The instruments built at Penn were designed to detect single-gamma emitters; therefore, their research and clinical applications were limited to the investigation of simple functions such as breakdowns of the blood-brain barrier in disorders such as brain tumors and cerebral infarcts. The instruments manufactured in the late 1960s and the early 1970s were also designed to image only the brain and not the other organs -- a limitation that was dictated by the technical difficulties encountered at the time.
Collaboration between investigators from nuclear medicine and the Cerebrovascular Research Center at the University of Pennsylvania resulted in great interest in quantitative measurement of regional cerebral function such as blood flow and blood volume. Although these attempts were implemented successfully, it became clear that synthesizing biologically important compounds with single-gamma-emitting radionuclides like technetium and iodine was a major challenge. As a result, other avenues began to be explored in an effort to overcome these limitations.
Deoxyglucose emerges
By the early 1970s, Louis Sokoloff and colleagues from the National Institutes of Health (NIH) and Martin Reivich from the University of Pennsylvania had clearly shown that the beta-emitting deoxyglucose-14C (DG) could be successfully utilized to map regional brain metabolism, which was later proven to correlate well with local function. These investigators were able to show that DG crosses the blood-brain barrier, and is phosphorylated by the hexokinase system to DG-6-phosphate similarly to glucose.
However, unlike glucose-6-phosphate, which further metabolizes to C02 and H20, DG-6-phosphate remains intact in the tissue for an extended time. This unique metabolic behavior makes radiolabeled deoxyglucose an excellent candidate for mapping regional function in the brain and other organs.
Since 14C is a beta-emitting radionuclide, optimal assessment of its distribution can be revealed by a technique called autoradiography. In animal experiments, 40-45 minutes after the intravenous administration of 14C-DG, slices of the brain were exposed to radiographic films to reveal the beta particles emitted for a period of time. The film was then processed to capture the regional distribution of the compound with exquisite detail.
Following successful demonstration of 14C-DG as a metabolic tracer, collaboration between NIH and Penn investigators resulted in defining and measuring parameters essential for calculating regional metabolic rates for glucose in various structures in the brain.
By the early 1970s, this powerful research technique had been adopted worldwide as an important tool for assessing regional brain function in a variety of physiological and pathological states, and in different animal models.
It became increasingly clear that employing the noninvasive DG method for the investigation of brain function in healthy and diseased states in humans would substantially advance our knowledge of neuropsychiatric disorders.
First label, then use
In late 1973 -- the same year that x-ray computed tomography was introduced by Hounsfield -- Martin Reivich, director of the Cerebrovascular Research Center at the Hospital of the University of Pennsylvania, David Kuhl, then director of nuclear medicine, and Abass Alavi, a junior staff member in nuclear medicine, discussed the possibility of labeling DG with a gamma-emitting radionuclide for in vivo imaging by an appropriate instrument.
These investigators were certain that only positron-emitting radionuclides would be suitable for this purpose. To select an appropriate label for DG, they consulted Alfred Wolf, an organic chemist at Brookhaven National Laboratory (BNL) in Upton, NY, who had developed a great interest in synthesizing positron-emitting compounds.
At a joint meeting of investigators from BNL and Penn in December 1973, Wolf suggested that fluorine-18 rather than carbon-11 should be pursued as an appropriate option, due to its relatively long half-life and its low positron energy. The long life of fluorine-18 was also attractive for shipment of the compound from BNL to Penn, where the group had planned to conduct the first tomographic studies in humans.
At the conclusion of the meeting, Wolf expressed his and his group's great desire to work on this project. He also made it clear that he wanted to be a close research collaborator with Penn investigators after the synthesis had been accomplished.
Within two years NIH funding had been secured, resulting in the establishment of a PET center at Penn to initiate the project. Meanwhile, Tatsuo Ido had joined Wolf's lab as a visiting postdoctoral fellow from Japan and was assigned to the project. Ido became the author of the first paper describing the synthesis of this compound.
By 1975, FDG was successfully synthesized at BNL. Although the initial yield was low, it was sufficient to plan for human studies. The BNL group was also able to synthesize carbon-14 FDG, which was shown by Sokoloff and colleagues to have a similar behavior to that of carbon-14 DG in in vivo experiments carried out by NIH.
In addition, all the required steps were taken to ensure the product could be safely prepared for human studies. Soon thereafter, an investigational new drug application was filed with the FDA in preparation for the administration of FDG to humans.
In anticipation of the human experiments, Penn investigators had assembled a set of high-energy collimators for the university-designed-and-built Mark IV scanner so it could image the 511-keV gamma rays emitted as a result of positron decay. Until then, the scanner was capable of imaging only low-energy radionuclides such as technetium-99m and iodine-123. By midsummer 1976, researchers from BNL and Penn had decided that it was time for the first human studies.
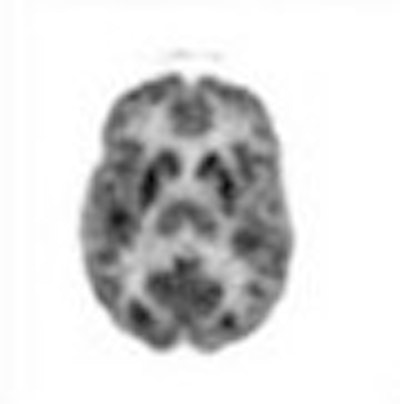
Thus, in August of that year, two normal volunteers each received a dose of FDG. The radionuclide was shown to concentrate in the brain by utilizing only one of the two gamma rays emitted from the annihilation of positron particles, instead of detecting the two gamma rays as a co-incident event (Fig. 1).
 |
| Fig. 1. The first whole-brain (planar) and tomographic FDG images of brain function in a normal volunteer reveal high concentration of the agent in cortical and subcortical gray matter. These images were acquired utilizing only one of the two 511-keV gamma rays emitted from the positron decay, as compared with the coincidence detection of appropriately designed modern instruments. The whole-brain scan was generated by an Ohio Nuclear Rectilinear scanner, while tomographic images were obtained with the Mark IV scanner, which was designed to examine central nervous system disorders. |
This was a gratifying outcome for investigators from both labs, who had worked so hard for so many years to achieve the goal of creating the first images of cerebral glucose metabolism in humans.
The quality of the images was poor, not comparable to that of scans acquired and reconstructed with modern instruments (Fig. 2).
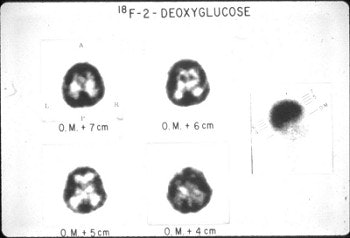
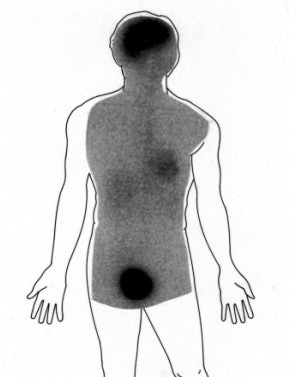
A whole-body image of the FDG distribution was also obtained in one subject using a dual-head gamma camera (Ohio Nuclear, Cleveland) that was also equipped with high-energy collimators for strontium-85 bone studies (Fig. 3). Uptake of FDG in the heart and significant renal excretion of the compound was demonstrated on this first human whole-body study. Obviously, the quality of whole-body images with FDG is substantially enhanced by instruments optimally designed for this purpose (Fig. 4).
The PET projects
Concurrent with the developments at the University of Pennsylvania and BNL, investigators at Washington University, directed by Michel TerPogossian and in collaboration with Michael Phelps and Edward Hoffman, had developed the first successful PET machine for optimal imaging of positron-emitting radionuclides in humans.
Soon thereafter, Gerd Muehllehner at Searle Radiographics (later purchased by Siemens Medical Solutions) successfully demonstrated the feasibility of employing two opposing scintillation cameras as coincident detectors to image positron-emitting radionuclides. This approach was later perfected while Muehllehner was a faculty member at Penn.
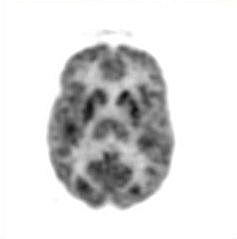 |
| Fig. 2. An FDG image of the brain obtained with a recently designed FDG-PET brain scanner acquired in June 2001. This image clearly demonstrates substantial technical improvements in the capability of instruments introduced over the past 25 years. |
In mid-1976, David Kuhl was recruited to UCLA as the director of nuclear medicine at that institution. By the fall of that year he had assembled an outstanding team of investigators to further explore the potential application of PET in disorders of the central nervous system and other organs.
At that time, UCLA was one of a few centers with a functioning cyclotron in a medical environment. A PET scanner, designed and built based upon principles established by Washington University's PETT III scanner, was soon installed at UCLA. As a result, investigators at the institution were able to image FDG uptake (the synthesis scheme was established with assistance from the BNL group) in the brain with an optimal instrument.
The group headed by Kuhl demonstrated the ability of FDG-PET imaging in mapping cerebral function in a variety of physiological and pathological states. In the meantime, the PETT III scanner designed and manufactured at Washington University was transferred to BNL for the purpose of conducting human studies.
The next phase of collaboration between BNL and University of Pennsylvania investigation was initiated when Al Wolf asked the research team from the latter institution to conduct FDG-based central nervous system projects at BNL. Every other week a research team, directed by Alavi, traveled to BNL to perform several interesting and important research projects, first in normal volunteers and later in patients.
 |
| Fig. 3. Whole-body images obtained following the acquisition of tomographic studies utilizing a dual-head Ohio Nuclear Rectilinear scanner. This scanner was equipped with high-energy collimators for performing strontium-85 bone scans. These first whole-body FDG images revealed uptake of the compound in the heart (in addition to the brain) and significant excretion through the kidneys. |
By 1979 the Penn group directed by Reivich and Alavi had established a PET center independent of BNL, but collaboration between the two institutions continued for more than a decade.
Cancer applications
The extraordinary power of functional imaging evidenced by the FDG-PET technique generated a great deal of interest in the scientific community. This led the NIH to establish several centers, including the University of Michigan, Johns Hopkins, Washington University, and the NIH campus at Bethesda, MD, in addition to Penn and UCLA, expanding the domain of research beyond what had been achieved with FDG.
Based on an observation made by Warburg in the 1930s that malignant cells utilize glucose preferentially over other substrates, Som and colleagues at BNL were able to demonstrate substantial concentration of FDG in tumor models in animals based on these principles, and for the first time, FDG was used by Dichiro and colleagues at the NIH to investigate metabolic activities of brain tumors in man at diagnosis and following treatment.
They were able to demonstrate that the degree of FDG uptake correlated with the grade of the tumor. It was also a predictor of outcome at diagnosis. More importantly, it was noted that FDG-PET was superior to contrast-enhanced CT and MRI in differentiating recurrent tumors from radiation necrosis.
Investigators from Penn (Jane and Abass Alavi) further confirmed these early observations, and since the mid-1980s FDG-PET has been widely used to examine patients with brain tumors specifically for the diagnosis of recurrent brain malignancies.
During most of the 1980s, the performance of whole-body imaging with PET was validated, and, by the early 1990s, its application as an effective modality was realized for this purpose.
Investigators from UCLA, and later from Duke and the universities of Michigan, Nebraska, and Heidelberg, Germany, were among the pioneers in demonstrating the efficacy of FDG-PET in the management of patients with a variety of malignancies. FDG-PET’s contributions included diagnosis, staging, monitoring treatment, and detecting recurrence of a variety of tumors.
The role of FDG-PET as the study of choice in differentiating benign from malignant nodules is unchallenged at this time. This imaging technique has substantially simplified the management of patients with solitary pulmonary nodules, and has enabled highly accurate staging of patients with lung cancer.
Detection of recurrent tumors by FDG-PET following surgery for colon cancer, as evidenced by elevated serum carcinoembryonic antigen (CEA) levels, has been revolutionary since CT and other anatomic imaging techniques fail to demonstrate the sites of disease in the majority of patients. FDG-PET imaging is not only very cost-effective in this setting, it is of great importance in providing an answer for a difficult clinical problem and has been well accepted by the clinical oncologists.
FDG-PET may completely replace other imaging techniques for the initial staging, restaging, and monitoring of the effects of treatment in patients with Hodgkin's and non-Hodgkin's lymphomas. The extraordinary sensitivity and specificity of FDG-PET allows the detection of disease activity in the lymph nodes and other organs with great precision. It is conceivable that in the near future, this modality will be used as the study of choice in the management of patients with lymphomas.
Similar statements can be made about the application of FDG-PET to other malignancies, including head and neck tumors, breast cancer, melanoma, ovarian cancer, mesothelioma, and possibly thyroid cancer and genitourinary tumors.
Current and future uses
Although FDG-PET can play a role in diagnosis, its major contribution has been in the accurate staging of cancer, in the assessment of treatment response, and above all in detecting recurrence following medical, radiation, and surgical therapies. Changes due to such treatments render structural techniques incapable of providing a definitive answer about the disease activity in many occasions.
As an effective technique for the assessment of myocardial viability, FDG-PET is well established, and is considered as the gold standard for this purpose. However, because of the success of conventional imaging with single-emitting radiopharmaceuticals, only in limited circumstances is PET requested for determination of myocardial viability.
Increasingly, FDG-PET imaging is being used to detect suspected orthopedic infections in failed prostheses, complicated fractures, and osteomyelitis. Detection of inflammatory processes including sarcoidosis, regional ileitis, and arthritis may allow applications of FDG-PET imaging for these challenging disorders and further enhance its clinical utility.
Also, early data from our laboratory and the Sloan Kettering Memorial Cancer Center demonstrate the feasibility of this technique in detecting atherosclerosis, which, following validation, may become an added domain for this exciting modality.
Challenges ahead
As we witness the rapid expansion of FDG-PET in the day-to-day practice of medicine around the world, the major difficulty being encountered by almost every group is the tremendous shortage of personnel who are properly trained in the discipline and are competent in performing various tasks associated with optimal use of this technology.
This applies to several categories of professionals whose contributions have been essential for the successful evolution of PET over the past 25 years. There is a great shortage of technical staff to manage cyclotron facilities, produce radionuclides, synthesize compounds, and perform the required quality control for human studies. There is also a great need for nuclear medicine technologists who are optimally trained to perform these studies.
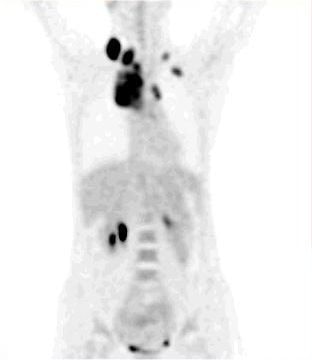 |
| Fig. 4. Whole-body FDG-PET projection images of a patient with a lymphoma reveal great detail about various structures included in the field of view. The high quality of these images demonstrates a substantial improvement in PET instrumentation, which has further enhanced effective utilization of FDG in a multitude of malignant and nonmalignant disorders. |
Above all, experienced and competent physicians who can provide this service in a competent manner in the community are very few and in great demand. Training in diagnostic radiology and conventional nuclear medicine is inadequate for interpretation of these complex, artifact-prone studies.
It may not be an exaggeration to consider FDG-PET images as some of the most difficult in the diagnostic discipline. It is not uncommon to spend 20 to 30 minutes examining a case so that one can confidently render an accurate assessment of the findings portrayed on the scan.
Knowledge of cross-sectional anatomy is helpful for optimal interpretation of FDG-PET images. There is a misconception that these studies must be interpreted by a radiologist, rather than by a competent nuclear medicine physician who is not fully trained in cross-sectional anatomic imaging techniques. Currently, most cases around the world are adequately and competently interpreted by nonradiologists, and this trend will continue in the foreseeable future. In fact, most PET specialists have mastered skills in comparing FDG-PET images with other diagnostic techniques such as CT and MRI when such comparisons have been necessary.
It is becoming quite clear that acquiring optimal skills to interpret FDG-PET images requires a great deal of training in this complex specialty. It would be unfortunate if sub-optimal services provided by untrained groups were to tarnish the image of the discipline as an effective modality.
Finally, it is quite evident that this type of service should be provided by centers with active cancer programs. Such centers should be able to refer at least 2-3 patients daily to PET facilities in order to ensure the financial viability of applying this complex technology.
We believe that fixed sites are preferable to mobile arrangements for this purpose. Fixed sites provide continuity of operations on a routine basis, which translates into technically optimal operation. The future of mobile units for this modality may be questionable at this time.
Surprisingly, FDG supply has adequately kept up with rapid expansion of this technique and does not appear to be a source of difficulty at this time. It is not clear whether future regulatory issues will limit FDG distribution within and across states.
Molecule of the century
In conclusion, FDG-PET increasingly plays a major role in the management of patients with a variety of disorders, especially cancer. This modality provides an exciting opportunity to imaging specialists, but this opportunity is also associated with serious challenges.
The imaging community must make every effort to be certain that this modality is appropriately utilized and managed so that, in the end, patients with difficult and challenging problems will benefit from its capabilities.
It is important to note that FDG-PET has made a lasting impact on our field. In fact, its routine use and acceptance by the medical community has served to rejuvenate nuclear medicine as a powerful discipline for the 21st century.
In the near future, the number of FDG scans performed in most active nuclear medicine services may exceed that of all other procedures performed in most laboratories. It is therefore quite fitting to applaud Dr. Henry Wagner for naming FDG the "Molecule of the 20th Century," because of its unique and unparalleled impact on the evolution of nuclear medicine.
By Dr. Abass Alavi and Dr. Martin ReivichAuntMinnie.com contributing writers
April 16, 2002
Copyright © 2002 AuntMinnie.com




















