Israeli gamma camera developer Spectrum Dynamics of Tirat Hacarmel has received clearance from the U.S. Food and Drug Administration for its D-SPECT cardiac system with BroadView imaging technology.
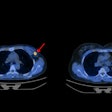
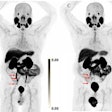
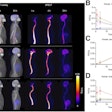
D-SPECT reduces acquisition time of nuclear cardiology imaging exams while also improving sensitivity and resolution, according to the company. Its BroadView technology increases sensitivity by collecting photons with larger collection angles, using a solid-state detector design and proprietary image reconstruction algorithms. D-SPECT can acquire patient data 10 times faster than a sodium iodide-based system, according to Spectrum, and provides twice the resolution, which the company hopes will reduce required patient radiation doses and allow clinicians to tailor nuclear cardiology exams to their patients.
Spectrum Dynamics plans to begin shipments in 2007, once clinical studies are completed.
By AuntMinnie.com staff writers
November 27, 2006
Related Reading
Road to RSNA, Spectrum Dynamics, November 15, 2006
Spectrum Dynamics taps Gurewitz, August 4, 2006
Spectrum Dynamics prepares launch of D-SPECT cardiac gamma camera, June 19, 2006
Spectrum Dynamics shows D-SPECT system, June 7, 2006
Copyright © 2006 AuntMinnie.com