Dear Molecular Imaging Insider,
This issue of the Insider offers a look at FDG-PET/CT's ability to predict a breast cancer patient's response to treatment after two cycles of neoadjuvant chemotherapy, helping physicians decide on the next course of action.
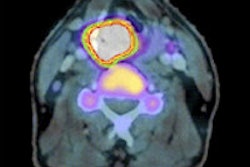
Researchers from the University of Düsseldorf in Germany compared maximum standardized uptake (SUVmax) values among patients who responded well to neoadjuvant chemotherapy and those who did not. Women who responded best to treatment saw a significant decrease in SUVmax between their FDG-PET/CT before neoadjuvant chemotherapy and a follow-up scan after two rounds of therapy.
Learn more in our Insider Exclusive, which is available to you before the other members of AuntMinnie.com.
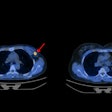
PET also recently showed its prowess when combined with the radiotracer F-18 fluorothymidine (FLT). In a study from the Netherlands, change in FLT uptake on PET during early radiotherapy or chemoradiotherapy was a strong indicator of long-term outcomes of head and neck cancer patients. Better three-year disease-free survival was associated with an SUVmax decrease of at least 45% and a gross tumor volume decrease of at least 31% during the first two weeks of treatment, the group found.
Gamma Medica has a better handle on its future now that the company has gone from Chapter 11 bankruptcy to having its molecular breast imaging (MBI) and preclinical imaging business segments bought by two suitors. The company's LumaGem MBI unit was sold to Imaging Acquisition for $3.1 million, while Northridge Trimodality Imaging acquired the preclinical business for $2.5 million.
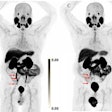
Regulatory changes also may be on the horizon for PET advocates. The U.S. Centers for Medicare and Medicaid Services (CMS) has proposed removing its longstanding requirement that FDG-PET scans of patients with solid tumors be reported to a national data registry. If the proposal is approved, PET providers will no longer have to report data to the National Oncologic PET Registry as a condition of reimbursement. On the other hand, CMS has only agreed to pay for one scan.
Last month, CMS removed a national noncoverage determination for radiopharmaceuticals with oncology PET imaging. However, CMS was not swayed by lobbying efforts to include cardiac and neurological applications in the decision. CMS concluded that unless there is a specific national coverage determination, local Medicare Administrative Contractors may determine coverage within their respective jurisdictions for oncologic imaging.
Be sure to visit the Molecular Imaging Digital Community during the week of April 14: AuntMinnie.com will provide daily updates from the American Roentgen Ray Society annual meeting in Washington, DC.