Blue Earth Diagnostics announced that its new drug application (NDA) filing for the PET agent fluciclovine has been accepted by the U.S. Food and Drug Administration (FDA) for priority review.
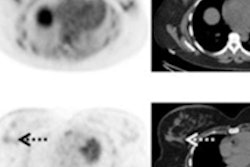
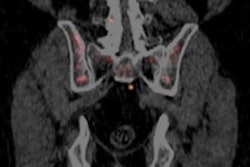
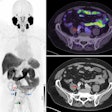
The firm is seeking U.S. marketing approval of fluciclovine (F-18) for lesion detection and localization for prostate cancer patients experiencing biochemical recurrence. Fluciclovine is a synthetic amino acid investigational PET radiopharmaceutical being studied by Blue Earth in the imaging of various cancers, with its lead product being in prostate cancer. The NDA submission for fluciclovine is based on data from more than 700 prostate cancer patients, most with biochemical recurrence and some with high-risk primary disease, imaged in the U.S., Norway, and Italy.
If approved, Siemens Healthcare subsidiary PETNet Solutions will manufacture, distribute, and sell the radiopharmaceutical in the U.S.