A new, relatively long-lived PET tracer can safely and effectively image patients with glioblastoma, according to research presented at the annual meeting of the Society of Nuclear Medicine and Molecular Imaging (SNMMI). The tracer also shows potential as a radiotheragnostic agent.
A multinational team of researchers reported on their experience with copper-64-EBRGD (Cu-64-EBRGD). The tracer utilizes the peptide sequence Arg-Gly-Asp (RGD) to specifically target the cell surface receptor αvβ3 integrin, which is overexpressed in glioblastomas. Clearance is slowed using Evans Blue (EB) dye, which is bound to RGD and significantly enhances target accumulation and retention, according to the authors. Adding the Cu-64 label to EBRGD enables the tracer to serve as a persistent, high-contrast diagnostic method in glioblastoma patients, according to the researchers.
At SNMMI 2020, the authors presented the results from a first-in-human study of EBRGD, reporting that the tracer had a favorable pharmacokinetic and dosimetry profile in patients with glioblastoma. It offered superior high-contrast imaging, visualizing tumors that express low or moderate levels of αvβ3 integrin with high sensitivity, they said.
The researchers first assessed the technique by performing whole-body Cu-64-EBRGD PET/CT exams on three healthy volunteers. In addition to collecting safety data after one day and then one week, they drew regions of interest, obtained time-activity curves, and calculated dosimetry.
Two patients with recurrent glioblastoma also received Cu-64-EBRGD PET/CT studies. In these patients, seven sets of brain PET and PET/CT scans were obtained over two consecutive days. The researchers also calculated tumor-to-background ratios for the target tumor lesion and normal brain tissue. Immunohistochemical staining of tumors samples was performed after surgical treatment one week following administration of the radiotracer.
They reported that Cu-64-EBRGD was well-tolerated by patients, with no adverse symptoms immediately or up to one week after administration. Also, the mean effective dose of the radiopharmaceutical was very similar to the effective dose of an 18F-FDG PET scan.
In the recurrent glioblastoma patients, the Cu-64-EBRGD injection showed high accumulation at the tumor, along with continuously increased tumor-to-background contrast over time. The postoperative pathology revealed glioblastoma rated as grade IV on the World Health Organization scale, and immunohistochemical staining demonstrated moderate expression of the αvß3 integrin, according to the researchers
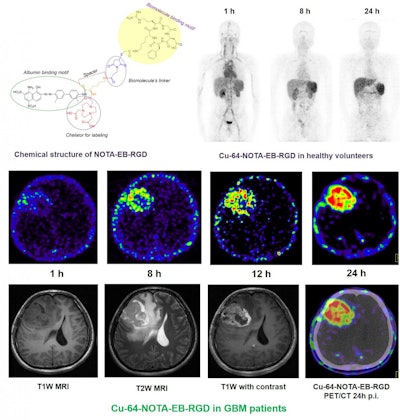 Maximum-intensity projection PET images of a healthy human volunteer injected with Cu-64-NOTA-EBRGD at 1, 8, and 24 hours after injection. Also, axial MRI and PET slices of glioblastoma patient injected with 64Cu-NOTA-EBRGD at different time points after injection. Images courtesy of Dr. Jingjing Zhang, PhD, et al, of Peking Union Medical College Hospital in Beijing, China, and Xiaoyuan Chen, PhD, et al, of the U.S. National Institute of Biomedical Imaging and Bioengineering (NIBIB).
Maximum-intensity projection PET images of a healthy human volunteer injected with Cu-64-NOTA-EBRGD at 1, 8, and 24 hours after injection. Also, axial MRI and PET slices of glioblastoma patient injected with 64Cu-NOTA-EBRGD at different time points after injection. Images courtesy of Dr. Jingjing Zhang, PhD, et al, of Peking Union Medical College Hospital in Beijing, China, and Xiaoyuan Chen, PhD, et al, of the U.S. National Institute of Biomedical Imaging and Bioengineering (NIBIB)."In this study, we have demonstrated a potential radiotheranostic agent that is safe, sensitive, and highly selective in humans, which infers a future diagnostic tool and breakthrough targeted radiotherapy for glioblastoma patients," said Dr. Jingjing Zhang, PhD, of Peking Union Medical College Hospital in Beijing, China, in a statement. "We believe this innovative use of Cu-64-EBRGD will significantly improve therapeutic efficacy and patient outcomes."
As lutetium-177, yttrium-90, or actinium-225 could be substituted for copper-64, Cu-labeled EBRGD represents a viable model for therapeutic applications, according to Dr. Deling Li of Beijing Tiantan Hospital.
"We are currently studying the Lu-177 homolog to treat glioblastoma and other αvβ3 integrin-expressing cancers, including non-small cell lung, melanoma, renal and bone, and hope to build on the current wave of radiotherapies like Lu-177 DOTATATE," Li said in a statement from the SNMMI.